Figure 3.
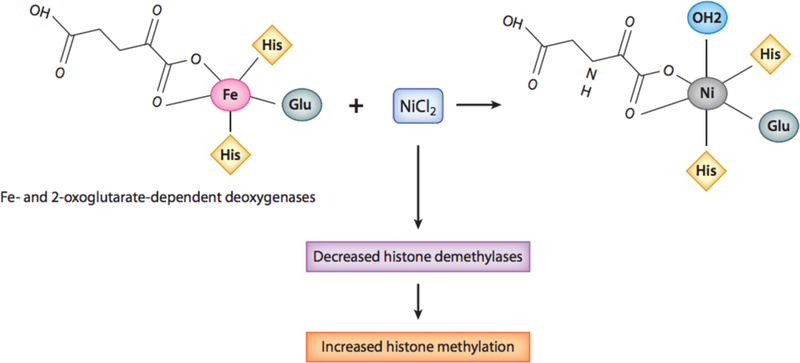
Model illustrating NiCl2 displacing Fe in 2-oxoglutarate-dependent oxygenases. Ni can displace Fe from its active site of Fe-2-oxoglutarate-dependent deoxygenases, which consists of two histidines and a carboxylate acid facial triad. Upon Fe displacement, Ni ions are able to coordinate with the same ligands as Fe, except that Fe is pentacoordinated, allowing for oxygen to bind, whereas Ni is hexacoordinated, resulting in an inactive enzyme. Abbreviations: Cl, chlorine; Fe, iron; Glu, glutamic acid; His, histidine; Ni, nickel.
