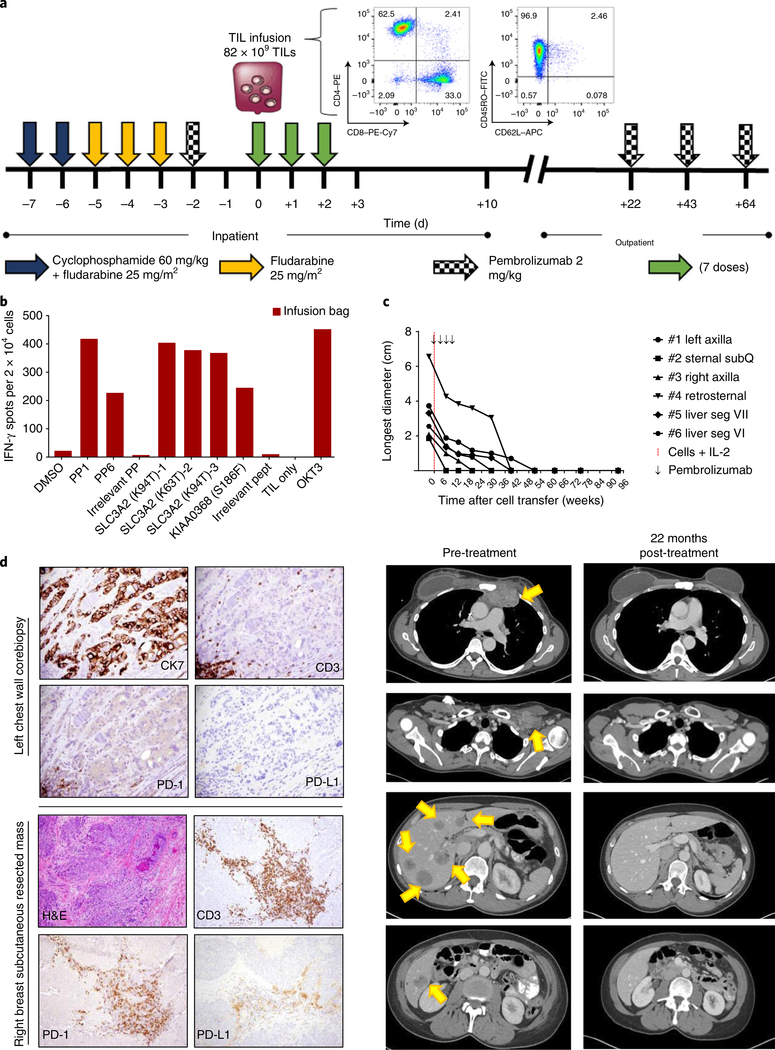
Fig. 2 |. Adoptive transfer of autologous TILs targeting immunogenic tumor mutations mediated tumor regression.
a, Treatment schema, with characteristics of the infusion product. Initial gating for flow cytometry analysis was done on live CD3+ cells. b, Interferon (IFN)-γ production, as determined by ELISPOT assay, showing that the infusion product, consisting of TILs expanded from fragments 8, 12 and 13 maintained their reactivity to mutSLC3A2 and mutKIAA0368. c, Top, response curves of target lesions (tumor size measurements). All lesions resolved 1 year after TIL transfer, and the patient continued to demonstrate complete response 22 months after cell infusion and 20 months after the last dose of pembrolizumab. Bottom, cross-sectional imaging was obtained 1 week before cell infusion (pre-treatment) and 22 months after infusion (22 months post-treatment). Arrows indicate target lesions (from top to bottom: retrosternal mediastinum, left axilla with clinical brachial plexopathy and compressed axillary vein, and multiple liver segments). SubQ, subcutaneous. d, Top, images showing pre-treatment tumor biopsy of a left chest wall mass (present at time of treatment), demonstrating ductal breast adenocarcinoma with scattered peripheral PD-1+ lymphocytes and few intratumoral lymphocytes (magnification: 40× ). Bottom, images showing the subcutaneous tumor that served as the source of TILs, with intratumoral PD-1+ lymphocytes and PD-L1+ stroma. Tumor cells were negative for PD-L1 expression (magnification: H&E-stained images, 10×; CD3-, PD-1- or PD-L1-stained images, 20× ).