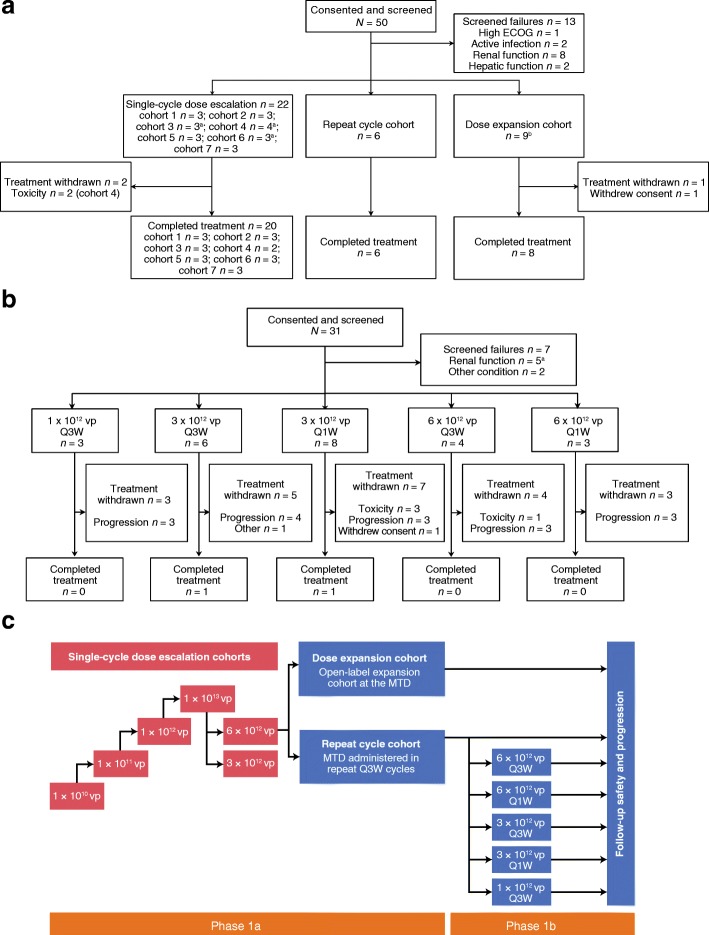
Fig. 1.
Patient disposition and study design. The phase 1 single-cycle dose escalation and dose expansion stages of EVOLVE were designed to determine the MTD of enadenotucirev, given in one cycle (one administration on days 1, 3, and 5 and an end-of-study visit 56 days after the last administration of enadenotucirev). a The phase 1a repeat cycle stage comprised up to four 21-day cycles and an end-of-study visit 28 days after the last administration of enadenotucirev. b The phase 1b component was initiated to select a suitable schedule and dose for repeat cycles of enadenotucirev in patients with mCRC or UCC. Phase 1b comprised up to six 21-day cycles, ending with an end-of-study visit 28 days after the last administration of enadenotucirev. Please refer to the study design and dosing and patient enrollment sections of the methods and patient demographics section of the results for full details of the enrollment into this phase. c Overall study design of the EVOLVE study. CRC colorectal cancer, ECOG Eastern Cooperative Oncology Group, EVOLVE EValuating OncoLytic Virus Efficacy, IV intravenous, mCRC metastatic colorectal cancer, MTD maximum tolerated dose, Q1W weekly schedule, Q3W 3-weekly schedule, UCC urothelial cell carcinoma, vp viral particle(s). aOne participant later received one additional cycle of treatment. bThree participants received one or more additional cycles of treatment. cOne participant also had inadequate bone marrow function