Abstract
Klippel-Feil syndrome is a congenital malformation characterized by the fusion of at least 2 cervical vertebrae. It may occur in association with other clinical syndromes and disorders.
We describe a case of prenatal diagnosis of a Klippel-Feil syndrome with Dandy-Walker malformation, and spina bifida, proved by ultrasound examination. A postmortem x-ray and autopsy were performed in a female fetus of 16 + 6 weeks of gestation: several malformations have been discovered.
To the best of our knowledge, no similar cases have been reported in the medical literature. This case report underscores the importance of a careful ultrasound screening during pregnancy for an adequate diagnostic and therapeutic management.
Keywords: Klippel-Feil syndrome, Dandy-Walker malformation, Spina bifida
Introduction
Klippel-Feil syndrome (KFS) is a genetic malformation characterized by a complex of osseous and visceral anomalies. It can be diagnosed at birth, but mild cases may go undiagnosed until symptoms worsen [2]. It may occur in association with other anomalies (eg, nervous system) [7].
We report a case of KFS, Dandy-Walker malformation (DWM), and spina bifida, aimed at describing its clinical features and the importance of an early diagnosis.
Clinical report
A 33-year-old woman, gravida 2 para 0, was referred to the Unit of Genetics, University of Sassari, Italy, at 16 + 5 weeks of gestation for the evaluation of a diagnosis of spina bifida detected by ultrasound.
An amniocentesis was immediately performed and the fetal karyotype of the female fetus resulted normal.
A more accurate ultrasound examination was performed at 16 + 6 weeks of gestation; this investigation was conducted through the 3 classical axial sections used to estimate the brain anatomy since the second trimester: transthalamic, transventricular, and transcerebellar planes [14].
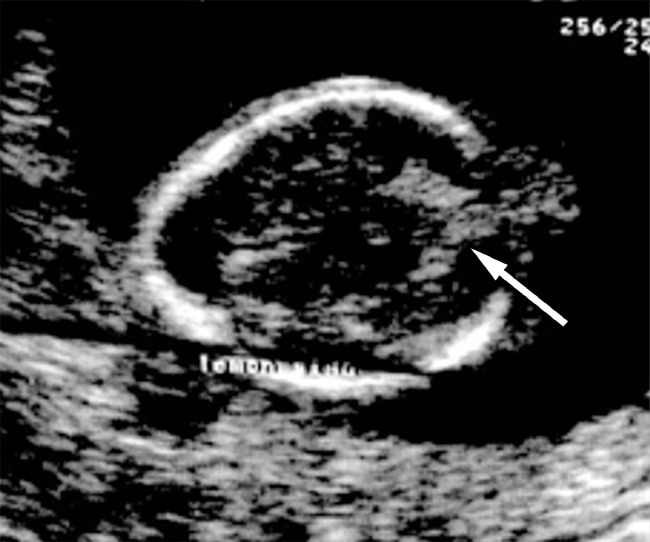
Fetal biometric measurements, such as biparietal diameter and occipitofrontal diameter, were computed from a cross-section of the fetal head at the level of the thalamus and cavum septi pellucidi. Both thalami and cavum septi pellucidi seemed normal. A scalloping of the frontal bones (“lemon sign”) was detected through the transventricular plane. No intracranial signs of hydrocephalus were observed. Following the posterior rotation of the probe (ie, >30 degrees), it was found an encephalocele of the posterior fossa and the elongation and downward displacement of the cerebellar hemispheres (“banana sign”). The concomitant agenesis of the cerebellar vermis poses the suspicion of a DWM [13] (Fig. 1).
Fig. 1.
Ultrasound scan reveal encephalocele of the posterior fossa, banana sign (arrow), lemon sign, and agenesis of the cerebellar vermis.
Through a sagittal view of fetal head, it was showed the corpus callosum and a hyperextension of the fetal head, which did not change during fetal movements.
The detailed ultrasound examination of the fetal spine proved the fusion of 5 cervical vertebrae (C1-C5) into a single block, providing evidence of a KFS.
Ultrasound confirmed a spinal meningocele in the thoracolumbar region: cross-sectional images showed abnormal ossification centers of the vertebral bodies and posterior processes.
The upper extremities were found to be normal, while a talipes equinovarus, commonly known as club foot, was detected at the right lower extremity.
Echocardiography proved the presence of a ventricular septal defect. No other fetal anomalies were found. After a genetic counseling, the pregnant woman decided to have an abortion.
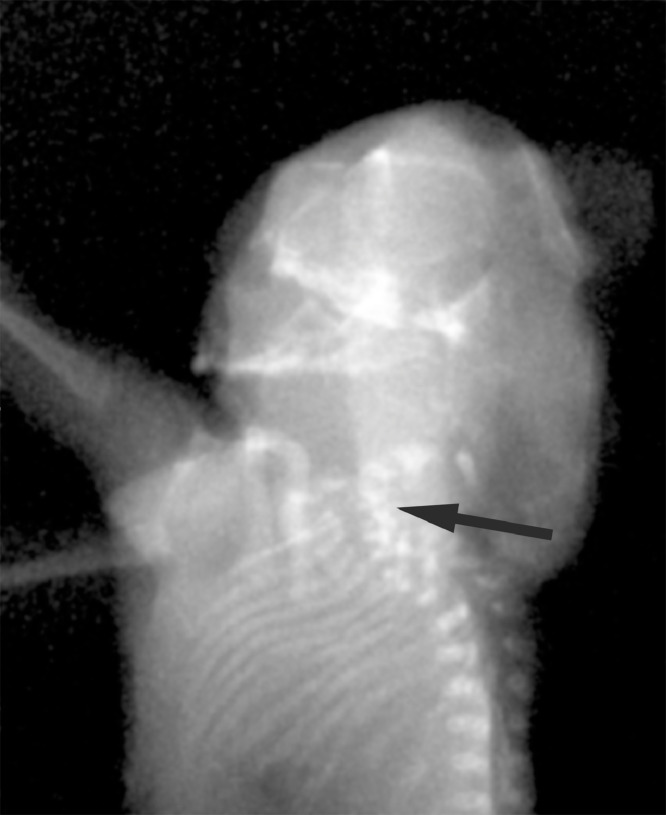
A postmortem x-ray examination was carried out. The posteroanterior and lateral views confirmed cervical spine fusion (i.e., C1-C5; Fig. 2), including a hemivertebra at C5.
Fig. 2.
Postmortem x-ray show cervical vertebrae fusion from C1 to C5 (arrow).
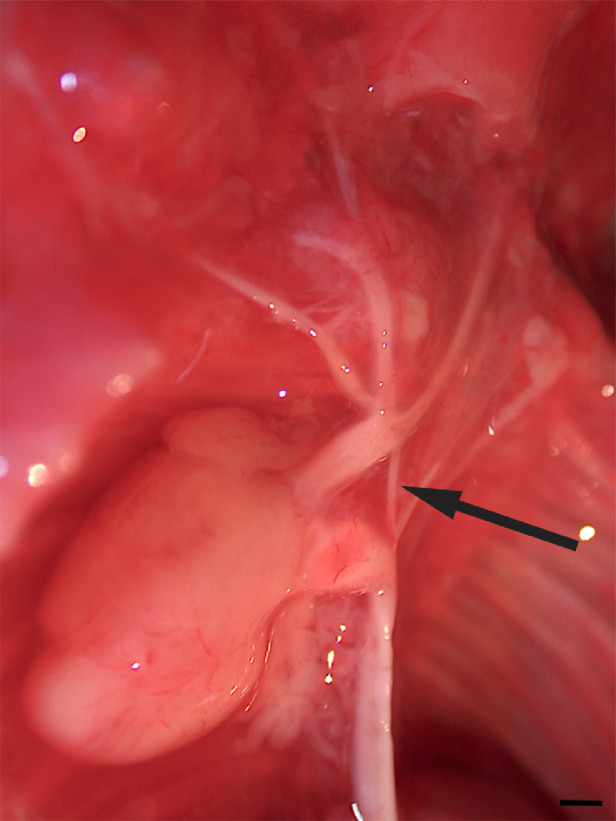
The macroscopic assessment of the fetus confirmed several abnormalities: neurocranial flattening, rachischisis, cervical vertebrae combined into a single block (C1-C5), C5 hemivertebra, and encephalocele containing brain tissue. Furthermore, a cerebellar vermis was not found. Fetal heart showed ventricular septal defect and aortic arch stenosis between the left common carotid and the left subclavian artery (Fig. 3).
Fig. 3.
Postmortem photograph show aortic arch stenosis between the left common carotid and subclavian (arrow). Scale bar = 0.5 mm.
Discussion
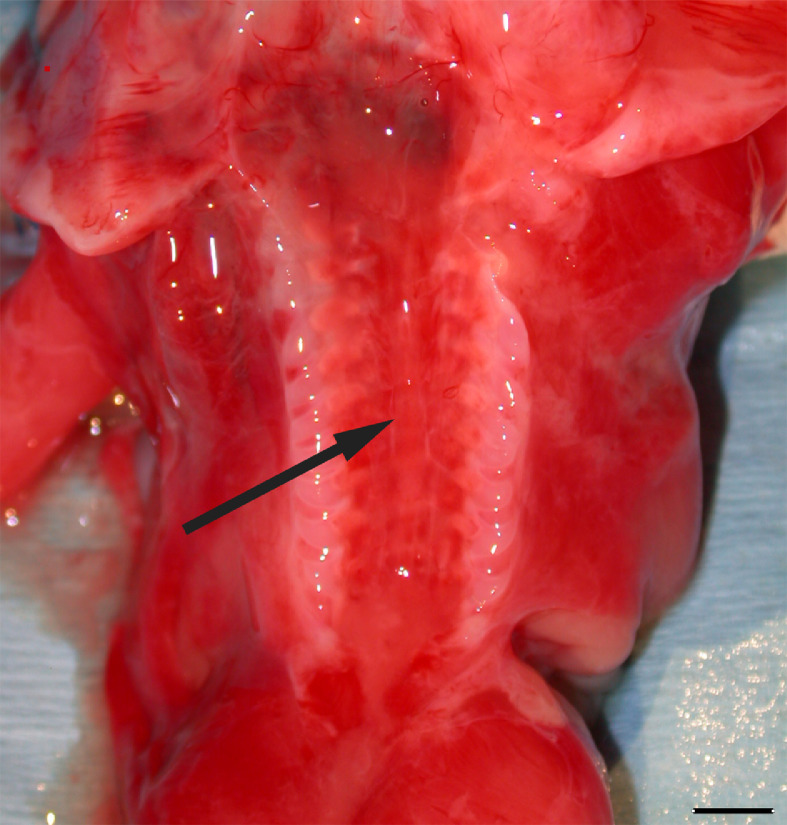
KFS, originally described in 1912 [8], is a rare skeletal congenital medical condition caused by the failed segmentation of the mesodermal somites [10] (Fig. 4).
Fig. 4.
Postmortem photograph show opening of vertebral canal (arrow) related to the lack of fusion of cervical, thoracic, and lumbar vertebral arches. Scale bar = 5 mm.
It can occur from the third to the eighth week of gestational development [10], [16], resulting in the fusion of 2 or more cervical vertebrae [4].
The estimated KFS's incidence ranges from 1 in 40,000 to 42,000 newborns worldwide, with a slight female predominance (female-to-male ratio: 3:2) [15].
The etiology is unknown, although several risk factors have been associated with congenital vertebral malformations [6], [12].
The majority of KFS cases occur sporadically, even if autosomal dominant or recessive, and X-linked forms have been described [3], [11].
Specific findings can be detected in KFS patients: short webbed neck, low posterior hairline, and severe restriction of cervical movements; however, their combination cannot always be found in KFS patients [15].
The Feil classification described 4 morphologic types: type I characterized by the massive fusion of cervical and upper thoracic vertebrae; type II characterized by the fusion of 1 or 2 cervical vertebrae; type III characterized by the fusion of both cervical and lower thoracic or lumbar vertebrae; type IV characterized by the cervical fusion and eye anomalies [12], [15].
This case report showed typical KFS features and, on the basis of Feil's classification, it fit type II KFS definition. The ultrasound screening, indeed, showed a fixed fetal head extension and a fusion into a single block of cervical vertebrae. Clinical findings were confirmed by the postmortem x-ray examination.
Cardiovascular anomalies, mainly septal defects, in association with KFS were described by other Authors [2]: the patient we described showed a ventricular septal defect and a preductal coarctation.
Furthermore, brainstem anomalies, congenital cervical stenosis, adrenal aplasia, ptosis, lateral rectus muscle paralysis, facial nerve paralysis, syndactyly, and diffuse or focal hypoplasia in upper extremities may also be detected [2], [7], as well as central nervous system abnormalities (eg, occipital cephalocele, Chiari I malformation, syrinx, microcephaly, and hydrocephalus) [7].
To our knowledge, there is only 1 report on the association between DWM and KFS [10]. DWM is a rare congenital disorder which consists of cerebellum anomalies, particularly agenesis of the cerebellar vermis, a cystic dilatation of the fourth ventricle, and an enlarged posterior fossa [1], [13]. DWM was suspected following the absence of the cerebellar vermis and the presence of an encephalocele, both confirmed by postmortem examination. Furthermore, in addition to the cerebellar abnormalities, our patient showed a neural tube defect: a meningocele in the thoracolumbar region.
Neural tube defects are congenital anomalies, characterized by the failure of the neural folds to fuse properly in the midline and form the neural tube [5]. Two major forms have been described: spina bifida aperta (SBA) and occulta. SBA may be referred to as either myeloschisis or myelomeningocele [9]. Being meningocele often described as a less severe variant of myelomeningocele, it can be considered a SBA.
Ultrasound evaluation and anatomical postmortem examination helped us to categorize the case as KFS type II, DWM, and spina bifida.
This case report highlights the importance of an early diagnosis of KFS, particularly if associated with other malformations such as a preductal coarctation.
KFS can show several degrees of severity; based on their prognosis following an accurate diagnosis, a team of healthcare professionals can adequately counsel parents and offer them the opportunity to decide about the future of the pregnancy.
Declarations of interest
None.
References
- 1.Arora R. Imaging spectrum of cerebellar pathologies: a pictorial essay. Pol J Radiol. 2015;80:142–150. doi: 10.12659/PJR.892878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejiqi R., Retkoceri R., Bejiqi H., Zeka N. Klippel–Feil syndrome associated with congential heart disease presentaion of cases and a review of the curent literature. Open Access Maced J Med Sci. 2015;3:129–134. doi: 10.3889/oamjms.2015.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke R.A., Singh S., McKenzie H., Kearsley J.H., Yip M.Y. Familial Klippel-Feil syndrome and paracentric inversion inv(8) (q22.2q23.3) Am J Hum Genet. 1995;57:1364–1370. [PMC free article] [PubMed] [Google Scholar]
- 4.Feil A. L'absence et la diminuation des vertebres cervicales (etude cliniqueet pathogenique); le syndrome dereduction numerique cervicales. Theses de Paris. 1919 [Google Scholar]
- 5.Fischer M., Stronati M., Lanari M. Mediterranean diet, folic acid, and neural tube defects. Ital J Pediatr. 2017;43:74. doi: 10.1186/s13052-017-0391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giampietro P.F., Raggio C.L., Blank R.D., McCarty C., Broeckel U., Pickart M.A. Clinical, genetic and environmental factors associated with congenital vertebral malformations. Mol Syndromol. 2013;4:94–105. doi: 10.1159/000345329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaman A., Kahveci H. Klippel-Feil syndrome and Dandy-Walker malformation. Genet Couns. 2011;22:411–415. [PubMed] [Google Scholar]
- 8.Klippel M., Feil A. Un cas d'absence des vertebres cervicales. Avec cage thoracique remontant jusqu'a la base du crane (cage thoracique cervicale) Nouv Iconog Salpetriere. 1912;25:223–250. [Google Scholar]
- 9.Mohd-Zin S.W., Marwan A.I., Abou Chaar M.K., Ahmad-Annuar A., Abdul-Aziz N.M. Spina Bifida: pathogenesis, mechanisms, and genes in mice and humans. Scientifica. 2017;2017 doi: 10.1155/2017/5364827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naikmasur V.G., Sattur A.P., Kirty R.N., Thakur A.R. Type III Klippel-Feil syndrome: case report and review of associated craniofacial anomalies. Odontology. 2011;99:197–202. doi: 10.1007/s10266-011-0004-7. [DOI] [PubMed] [Google Scholar]
- 11.Nouri A., Tetreault L., Zamorano J.J., Mohanty C.B., Fehlings M.G. Prevalence of Klippel-Feil Syndrome in a surgical series of patients with cervical spondylotic myelopathy: analysis of the prospective, multicenter AOSpine North America study. Global Spine J. 2015;5:294–299. doi: 10.1055/s-0035-1546817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samartzis D., Kalluri P., Herman J., Lubicky J.P., Shen F.H. “Clinical triad” findings in pediatric Klippel-Feil patients. Scoliosis Spinal Disord. 2016;11:15. doi: 10.1186/s13013-016-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadakamadla J., Kumar S., Mamatha G.P. Dandy-Walker malformation: an incidental finding. Indian J Hum Genet. 2010;16:33–35. doi: 10.4103/0971-6866.64936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The International Society of Ultrasound in Obstetrics and Gynecology Guidelines. Ultrasound Obstet Gynecol. 2007;29:109–116. [Google Scholar]
- 15.Tracy M.R., Dormans J.P., Kusumi K. Klippel–Feil syndrome: clinical features and current understanding of etiology. Clin Orthop Relat Res. 2004;2:183–190. [PubMed] [Google Scholar]
- 16.Yuksel M., Karabiber H., Yuksel K.Z., Parmaksiz G. Diagnostic importance of 3D CT images in Klippel-Feil Syndrome with multiple skeletal anomalies: a case report. Korean J Radiol. 2005;6:278–281. doi: 10.3348/kjr.2005.6.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]