FIGURE 1.
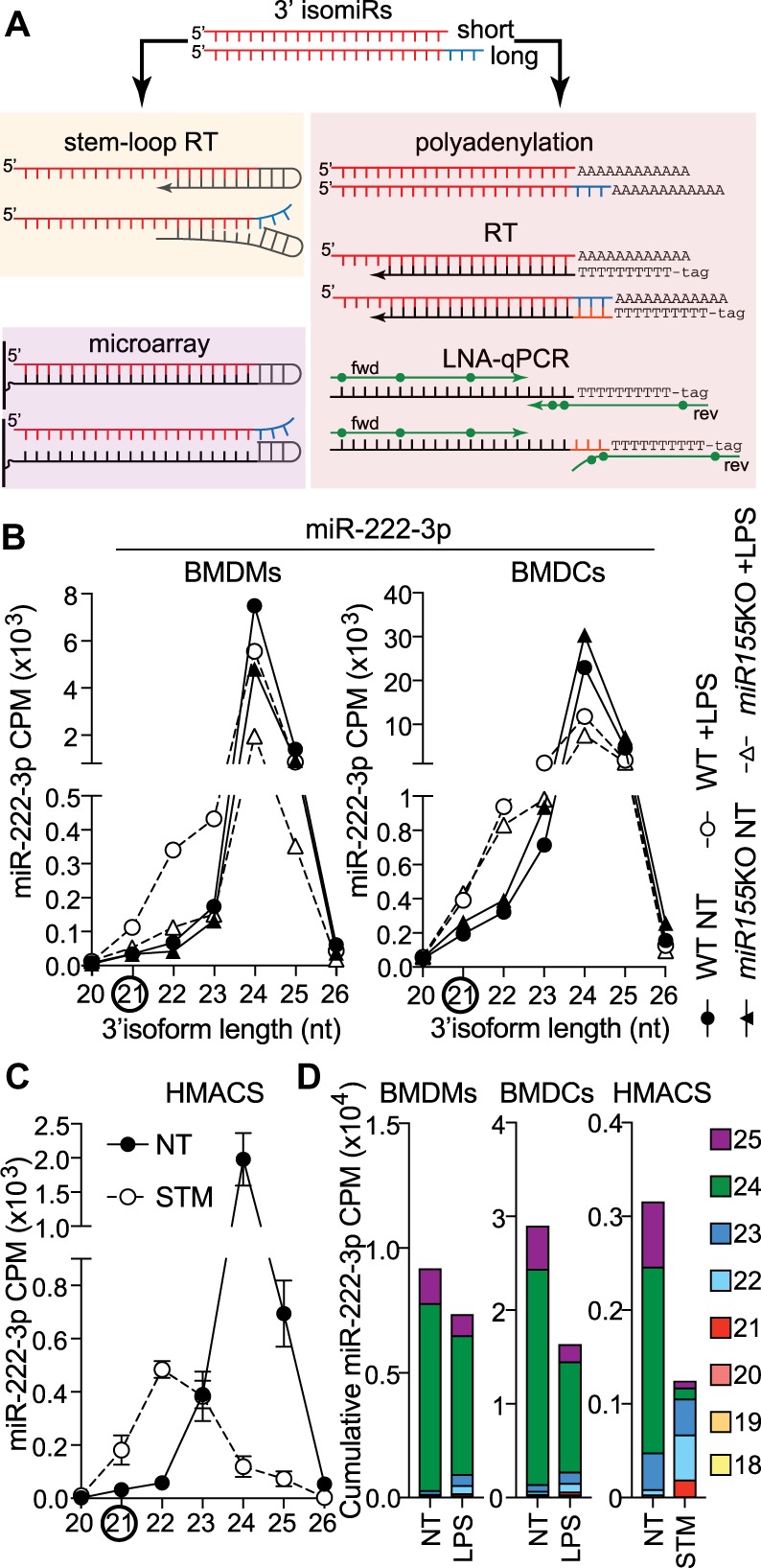
Bacterial-driven modulation of miR-222 isoforms in human and mouse macrophages. (A) Schematic representation of the impact of 3′-end length variation on stem–loop RT-qPCR, LNA-polyadenylation RT-qPCR, and Agilent microarray detection of mature miRNAs. In the case presented here, the assays are targeted to the shorter miRNA isoform, as seen with miR-222-3p. This directly impacts the binding of the reverse transcription stem–loop and efficiency of cDNA synthesis. Similarly, microarray loop-probes will not bind as well to longer isoforms. While it does not affect reverse transcription based on polyadenylation, the isoform selectivity impacts binding of the 3′-end of LNA reverse primer, hampering amplification. (B) Detailed analysis of normalized counts (CPM) for the 3′-end isoforms of miR-222 in primary macrophages (BMDMs) and dendritic cells (BMDCs) from wild-type (WT) and miR-155-deficient mice (Dueck et al. 2014). Cells were treated overnight with 200 ng/mL lipopolysaccharide (LPS) or nontreated (NT). Data shown are from one biological sample for each condition. (C) Detailed analysis of normalized counts (CPM) for the 3′-end isoforms of miR-222 in human monocyte-derived macrophages, infected for 24 h with Salmonella Typhimurium (STM) at an MOI of 5:1, compared to NT cells at 24 h (Pai et al. 2016). Data are averaged from six patients (mean ± standard error of the mean is shown). (D) CPM of the prevalent miR-222 3′-end isoforms from NT and LPS-treated WT BMDMs/BMDCs and STM-infected human macrophages were cumulated to reflect the overall impact of bacterial products on the pool of miR-222 molecules.
