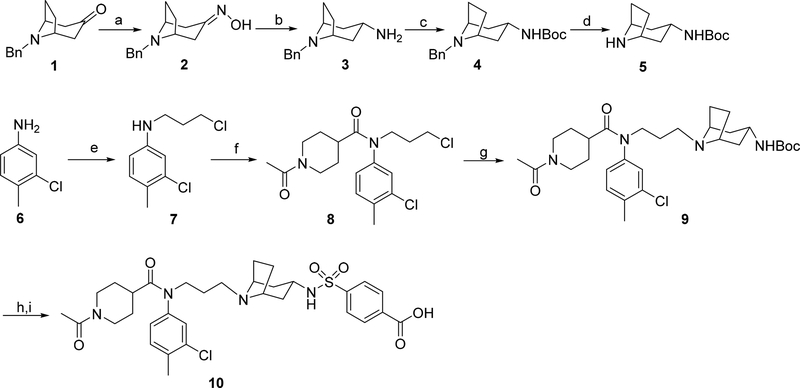
Figure 3.
Synthesis of the coreceptor inhibitor LJC240−COOH (10). Reagents and conditions: (a) NH2OH·HCl, NaHCO3, EtOH/H2O, rt; (b) Na, pentanol, reflux; (c) (Boc)2O, TEA, THF, rt; (d) 10% Pd/C, HCO2NH4, methanol, reflux; (e) 1-bromo-3-chloropropane, KI, TEA, DMF, rt; (f) 1-acetylpiperidine-4-carboxylic acid, SOCl2, TEA, DCM, rt; (g) 5, KI, K2CO3, MeCN, reflux; (h) HCl, methanol, rt; (i) 4-(chlorosulfonyl)benzoic acid, Na2CO3, dioxane/H2O, rt.