Abstract
Background
Clear cell adenocarcinoma of the lung (CCAL) is a rare diagnosis with poorly understood clinicopathological characteristics and disease progression.
Methods
A population cohort study was conducted using prospectively extracted data from the Surveillance, Epidemiology and End Results database for patients with histological diagnoses of CCAL. Propensity-matched analysis was performed for survival analysis.
Results
A total of 1,203 patients with CCAL were included. The median overall survival (OS) for all patients was 19.0 months (95% CI 16.0–22.0 months). Data for 1-, 3-, and 5-year OS were 58.7, 37.3, and 27.7%, respectively. Log-rank analysis showed that the prognoses of CCAL patients were better than those with non-CCAL adenocarcinoma after propensity-matched analysis (P<0.001). Cancer-directed surgery significantly improved median OS by almost 40 months (45.0 vs 5.0 months; P<0.01). Radiotherapy after surgery prolonged survival compared with patients who only received surgery (37.0 vs 17.0 months; P<0.01). Multivariate Cox analysis showed that older age (>65 years), larger lesions, and lymph node and distant metastases were independent prognostic factors for worse survival, while cancer-directed surgery was an independent protective factor. Five independent prognostic factors were identified and entered into the nomogram. The concordance index of the nomogram for predicting survival was 0.72 (95% CI 0.69–0.74). The calibration curves for the probability of 3-, 5-, and 10-year OS showed optimal agreement between nomogram prediction and actual observation.
Conclusion
CCAL is a rare pathology, and older age, larger lesions, metastases, and cancer-directed surgery were associated with prognosis. A prognostic nomogram was established to provide individual prediction of OS.
Keywords: clear cell adenocarcinoma of the lung, outcomes, SEER database, nomogram
Introduction
Lung adenocarcinoma featuring cells filled with clear cytoplasm as major tumor components is defined as primary clear cell adenocarcinoma of the lung (CCAL). Tumors with clear cell cytoplasm can occur in any organ, but renal clear cell carcinoma and clear cell adenocarcinoma of the female genital tract are the most common. Primary clear cell adenocarcinoma is an extremely rare pulmonary tumor that originates from perivascular epithelioid cells, accounting for ~0.3–3.4% of lung cancers.1 WHO classification of tumors identifies this disease as a subtype of mucus-producing adenocarcinomas.2 The first case was initially described by Liebow and Castleman in 1963,3 but only sporadic cases of CCAL have been reported until now.4–7
Most CCAL patients are clinically silent or asymptomatic, and few present with nonspecific symptoms similar to other primary pulmonary tumors, including dyspnea, cough, fever, chest pain, and hemoptysis, which are not distinctive enough to differentiate this condition from others.8,9 Therefore, early detection of CCAL is difficult and most cases are discovered incidentally with routine chest radiographs or CT scans. Clinical, imaging, and bronchoscopy findings are nonspecific and mimic other neoplasms, and a rare diagnosis of clear cell carcinoma is often established only during histopathological diagnosis. Bronchoscopy is essential for obtaining pathological specimens for definitive diagnosis, but due to its rarity, most CCAL studies have focused on case reports or series, and demographic and clinicopathological features of CCAL patients have not been characterized until now.
If possible, surgical removal should be performed once a CCAL diagnosis is made, even though the literature suggests poor prognosis for early metastasis or recurrence.10 Because there are few CCAL reports in the literature, case studies cannot suggest the best predictor of overall survival (OS). Thus, we performed a retrospective analysis of patients with CCAL registered in the Surveillance, Epidemiology and End Results (SEER) database and summarized the clinical characteristics and OS. Meanwhile, we established the prognostic nomogram to help clinicians to predict survival for individual CCAL patient.
Materials and methods
Participants
The Institutional Review Board of Punan Hospital of Pudong New District approved this study. The SEER program is supported by the National Cancer Institute and collects information, including cancer incidence and survival from 18 population-based cancer registries throughout USA, covering ~28% of the US population. All patients with a diagnosis of CCAL (ICD-O-3: 8310/3) according to the ICD-O-3/WHO 2008 between 1988 and 2013 were selected from the SEER database. Demographic features and the clinicopathological characteristics of each patient were collected, including age, sex, race, laterality, primary site, pathological differentiation, tumor size, lymph node metastases, distant metastases, SEER summary stage, and whether surgery and radiation were performed. The SEER database also reported cancer-specific survival, which was defined as the interval from diagnosis until death due to this kind of cancer or until the last follow-up.
Statistical analysis
Continuous data were compared using a Student’s t-test, and categorical data were compared using a Chi-squared test. To adjust for differences between CCAL and non-CAAL lung adenocarcinoma groups when analyzing prognoses, we identified patients diagnosed with non-CCAL lung adeno-carcinoma during same period from SEER database and performed propensity-score matching (PSM) analysis at a 1:1 ratio. The PSM model was based upon age, race, laterality, primary site, grade, tumor size, metastases, tumor stage, radiation, and surgery. Survival probabilities were estimated using a Kaplan–Meier method, and a log-rank test was used to assess any significant differences in OS stratified by each covariate. Cox proportional hazards models were used to analyze associations between clinicopathological characteristics with OS. HRs and 95% CIs were estimated using univariate and multivariable models. Multivariate analysis was performed to identify independent prognostic factors, and only these variables that were significantly associated with survival in univariate Cox analysis were included. The optimal cutoff levels of prognostic factors were determined by X-Tile Software. The prognostic nomogram, concordance index (C-index), and calibration curve were analyzed by R 3.1.3 (http://www.r-project.org) with the rms and survival packages. Statistical analysis was performed using the software MedCalc (Version 15.2.2; MedCalc Software, Mariakerke, Belgium) and SPSS 23.0 (IBM Corporation, Armonk, NY, USA), and P<0.05 was considered to be statistically significant.
Results
A total of 1,203 CCAL patients were included. Table 1 depicts the patients’ data. Most (59.9%) patients received cancer-directed surgery, and Table S1 depicts these traits of patients and their treatment options. Radiation was performed for 30.5% of cases (Table S2). CCAL patients with younger age, smaller lesions, no metastasis, as well as early stage had more likely to receive cancer-directed surgery. Conversely, CCAL patients with larger or metastatic lesions as well as late stage had more likely to receive radiation.
Table 1.
Characteristics of 1,203 patients with CCAL
| Characteristics | Number |
|---|---|
| Total patients | 1,203 |
| Age (years) | 65.2±10.9 |
| Gender | |
| Female | 588 (48.9%) |
| Male | 615 (51.1%) |
| Ethnicity | |
| White | 1,024 (85.2%) |
| Black | 127 (10.6%) |
| Others (American Indian, Alaska Native, Asian, Pacific Islander) | 50 (4.2%) |
| Unknown | 2 |
| Pathological differentiation | |
| Well | 46 (5.9%) |
| Moderate | 214 (27.5%) |
| Poor | 458 (58.9%) |
| Undifferentiated | 59 (7.7%) |
| Unknown | 426 |
| Summary stage | |
| Distant | 381 (32.6%) |
| Regional | 400 (34.3%) |
| Localized | 386 (33.1%) |
| Unstaged | 36 |
| Laterality | |
| Left | 490 (41.6%) |
| Right | 674 (57.2%) |
| Bilateral | 14 (1.2%) |
| Unknown | 25 |
| Primary site | |
| Main bronchus | 27 (2.5%) |
| Upper lobe, lung | 665 (61.8%) |
| Middle lobe, lung | 64 (5.9%) |
| Lower lobe, lung | 300 (27.9%) |
| Overlapping lesion of lung | 21 (1.9%) |
| Lung, not otherwise specified | 126 |
| Tumor stage | |
| T1 | 267 (29.7%) |
| T2 | 391 (43.5%) |
| T3 | 71 (7.9%) |
| T4 | 170 (18.9%) |
| Unknown | 304 |
| Tumor size (mm) | 39.9±23.7 |
| Lymph node metastases | |
| N0 | 570 (55.3%) |
| N1 | 122 (11.8%) |
| N2 | 260 (25.2%) |
| N3 | 79 (7.7%) |
| Unknown | 172 |
| Distant metastases | |
| Yes | 316 (27.4%) |
| No | 838 (72.6%) |
| Unknown | 49 |
| TNM stage | |
| I | 427 (39.7%) |
| II | 93 (8.7%) |
| III | 239 (22.2%) |
| IV | 316 (29.4%) |
| Unknown | 128 |
| Surgery | |
| Yes | 718 (59.9%) |
| No | 480 (40.1%) |
| Unknown | 5 |
| Surgery type | |
| Pneumonectomy | 44 (6.2%) |
| Lobectomy/bilobectomy | 534 (74.7%) |
| Partial resection | 137 (19.2%) |
| Unknown | 3 |
| Radiation | |
| Yes | 359 (30.5%) |
| No | 818 (69.5%) |
| Unknown | 26 |
Notes: Data presented as n, n (%), or mean ± SD. Percentage values have not been included for ‘Unknown’ data.
Abbreviation: CCAL, clear cell adenocarcinoma of the lung.
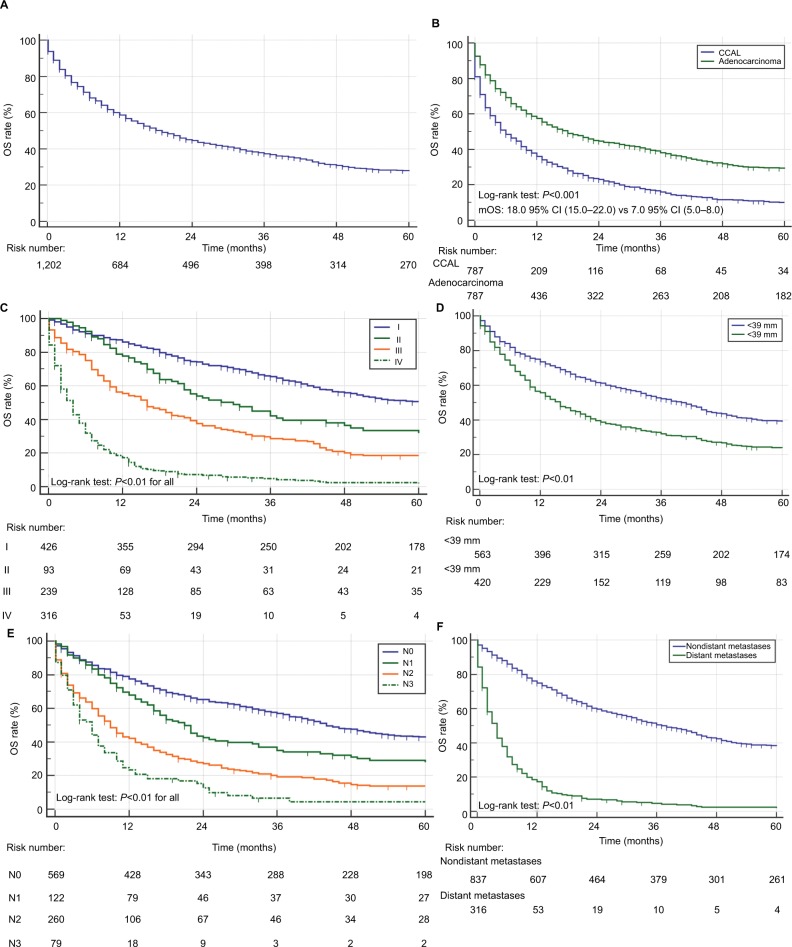
The median OS of all CCAL patients was 19.0 months (95% CI 16.0–22.0, Figure 1A). The overall 1-, 3- and 5-year survival rate was 58.7%, 37.3%, 27.7%. When we performed survival analysis in unmatched patients, the median OS (mOS) were 10 months in 266,652 lung adenocarcinoma patients, which was shorter than those in CCAL patients (Figure S1). A total of 787 CCAL patients were matched with 787 non-CCAL lung adenocarcinoma patients. As shown in Table S3, there were no significant differences in clinical characteristics after PSM analysis. Kaplan–Meier curves for survival and Log-Rank analysis showed that the prognoses of CCAL patient were significantly better than those with non-CCAL lung adenocarcinoma (Figure 1B). Multivariate Cox analysis revealed that CCAL could decrease the risk of death compared with non-CCAL lung adenocarcinoma (HR =0.56, 95% CI 0.50–0.63, P<0.01).
Figure 1.
OS for patients with CCAL.
Notes: (A) OS for 1,203 patients with CCAL. (B) OS for 787 CCAL patients with 787 matched lung adenocarcinoma patients. (C) OS for CCAL patients at different TNM stages. (D) OS for CCAL patients stratified by tumor size. (E) OS stratified by lymph node metastases. (F) OS stratified by distant metastases.
Abbreviations: CCAL, clear cell adenocarcinoma of the lung; mOS, median OS; OS, overall survival.
Kaplan–Meier curves and OS analysis stratified by clinical characteristics appeared in Table 2. Patients with more advanced disease stages had inferior outcomes as shown in Table 2 and in Figure 1C. The 1-, 3- and 5-year survival rates for patients with TNM I, II and III stage were 86.0%, 65.5%, 50.7% and 77.8%, 42.2%, 32.0% and 55.5% 28.7%, 18.6%, respectively, compared with just 17.2%–4.7%, 2.4% in patients with IV stage disease. Similarly, patients with later stages had significantly poorer prognoses than those with localized or regional disease of SEER summary stage (P<0.01 for both). Patients with larger lesions had worse prognoses (Figure 1D), and lymph node invasion was also an indicator of poor outcomes (Figure 1E). OS for CCAL patients with distant metastases was shorter as well (Figure 1F). Patients with lesions in the main bronchus had a worse prognosis compared with those whose lesions occurred in lung lobes, and younger subjects and female subjects had greater OS (Table 2).
Table 2.
Overall survival stratified by clinical characteristics and univariate Cox proportional hazard analyses for patients with CCAL
| Factor | Category | OS (months), median (IQR) | Univariate | |
|---|---|---|---|---|
| HR (95% CI) | P-value | |||
| Age (years) | <65 | 24.0 (21.0–32.0) | Reference | |
| >65 | 14.0 (11.0–18.0) | 1.44 (1.27–1.63) | <0.01 | |
| Gender | Female | 24.0 (20.0–31.0) | Reference | |
| Male | 14.0 (12.0–18.0) | 1.19 (1.05–1.35) | <0.01 | |
| Race | White | 19.0 (16.0–22.0) | Reference | |
| Black | 13.0 (10.0–24.0) | 1.06 (0.87–1.30) | 0.54 | |
| Others | 20.0 (16.0–28.0) | 0.82 (0.59–1.14) | 0.24 | |
| Pathological differentiation | Well | 39.0 (16.0–73.0) | Reference | |
| Moderate | 46.0 (36.0–63.0) | 0.82 (0.57–1.16) | 0.26 | |
| Poor | 22.0 (18.0–27.0) | 1.19 (0.86–1.67) | 0.29 | |
| Undifferentiated | 22.0 (15.0–43.0) | 0.99 (0.65–1.51) | 0.96 | |
| Summary stage | Localized | 55.0 (46.0–70.0) | Reference | |
| Regional | 28.0 (23.0–33.0) | 1.37 (1.17–1.61) | <0.01 | |
| Distant | 4.0 (3.0–5.0) | 5.96 (5.02–7.07) | <0.01 | |
| Laterality | Left | 20.0 (16.0–26.0) | Reference | |
| Right | 20.0 (17.0–23.0) | 0.99 (0.87–1.12) | 0.90 | |
| Bilateral | 3.0 (1.0–6.0) | 3.98 (2.33–6.79) | <0.01 | |
| Primary site | Main bronchus | 8.0 (2.0–10.0), | Reference | |
| Upper lobe, lung | 25.0 (21.0–31.0) | 0.46 (0.31–0.69) | <0.01 | |
| Middle lobe, lung | 24.0 (17.0–45.0) | 0.50 (0.31–0.80) | <0.01 | |
| Lower lobe, lung | 21.0 (16.0–30.0) | 0.51 (0.34–0.76) | <0.01 | |
| Overlapping | 21.0 (10.0–85.0) | 0.40 (0.21–0.75) | <0.01 | |
| Lymph node metastases | N0 | 44.0 (40.0–51.0) | Reference | |
| N1 | 21.0 (16.0–26.0) | 1.31 (1.06–1.63) | <0.01 | |
| N2 | 9.0 (7.0–11.0) | 2.25 (1.87–2.71) | <0.01 | |
| N3 | 6.0 (4.0–7.0) | 3.56 (2.44–5.21) | <0.01 | |
| Distant metastases | No | 38.0 (33.0–43.0) | Reference | |
| Yes | 4.0 (3.0–5.0) | 5.23 (4.48–6.10) | <0.01 | |
| TNM | I | 63.0 (50.0–76.0) | Reference | |
| II | 30.0 (22.0–45.0) | 1.30 (0.99–1.71) | 0.06 | |
| III | 16.0 (11.0–20.0) | 2.23 (1.86–2.67) | <0.01 | |
| IV | 4.0 (3.0–5.0) | 7.1 (5.95–8.55) | <0.01 | |
| Surgery | No | 5.0 (4.0–6.0) | Reference | |
| Yes | 45.0 (41.0–51.0) | 0.22 (0.19–0.25) | <0.01 | |
| Radiation | No | 27.0 (22.0–33.0) | Reference | |
| Yes | 10.0 (9.0–12.0) | 1.59 (1.39–1.82) | <0.01 | |
| Surgery type | Partial resection | 41.0 (27.0–53.0) | Reference | |
| Lobectomy/bilobectomy | 48.0 (43.0–60.0) | 0.81 (0.65–0.99) | 0.07 | |
| Pneumonectomy | 32.0 (16.0–107.0) | 0.85 (0.57–1.27) | 0.44 | |
| Tumor size (mm) | <39 | 40.0 (33.0–45.0) | Reference | |
| >39 | 16.0 (13.0–19.0) | 1.48 (1.29–1.71) | <0.01 | |
Abbreviations: CCAL, clear cell adenocarcinoma of the lung; OS, overall survival.
Figure 2A shows that cancer-directed surgery significantly improved the mOS for patients with CCAL (45.0 months vs 5.0 months, P<0.01), but there was no difference among different surgical methods (Table 2). PSM analysis for CCAL patients receiving radiation appeared in Table S4 and survival analysis showed patients who received radiation had longer survival (Figure 2B, 11.0 months vs 8.0 months, P=0.02). Also, PSM analysis for CCAL patients receiving radiotherapy after surgery appeared in Table S5 and radiotherapy after surgery could prolong survival compared with surgery alone (Figure 2C, 37.0 months vs 17.0 months, P=0.02). When stratified by TNM stage, longer survival occurred with TNM I, II, III, especially TNM III (mOS: 15.0 95% CI (8.0–25.0) vs 30.0 95% CI (19.0–62.0), P<0.01) (Figure S2).
Figure 2.
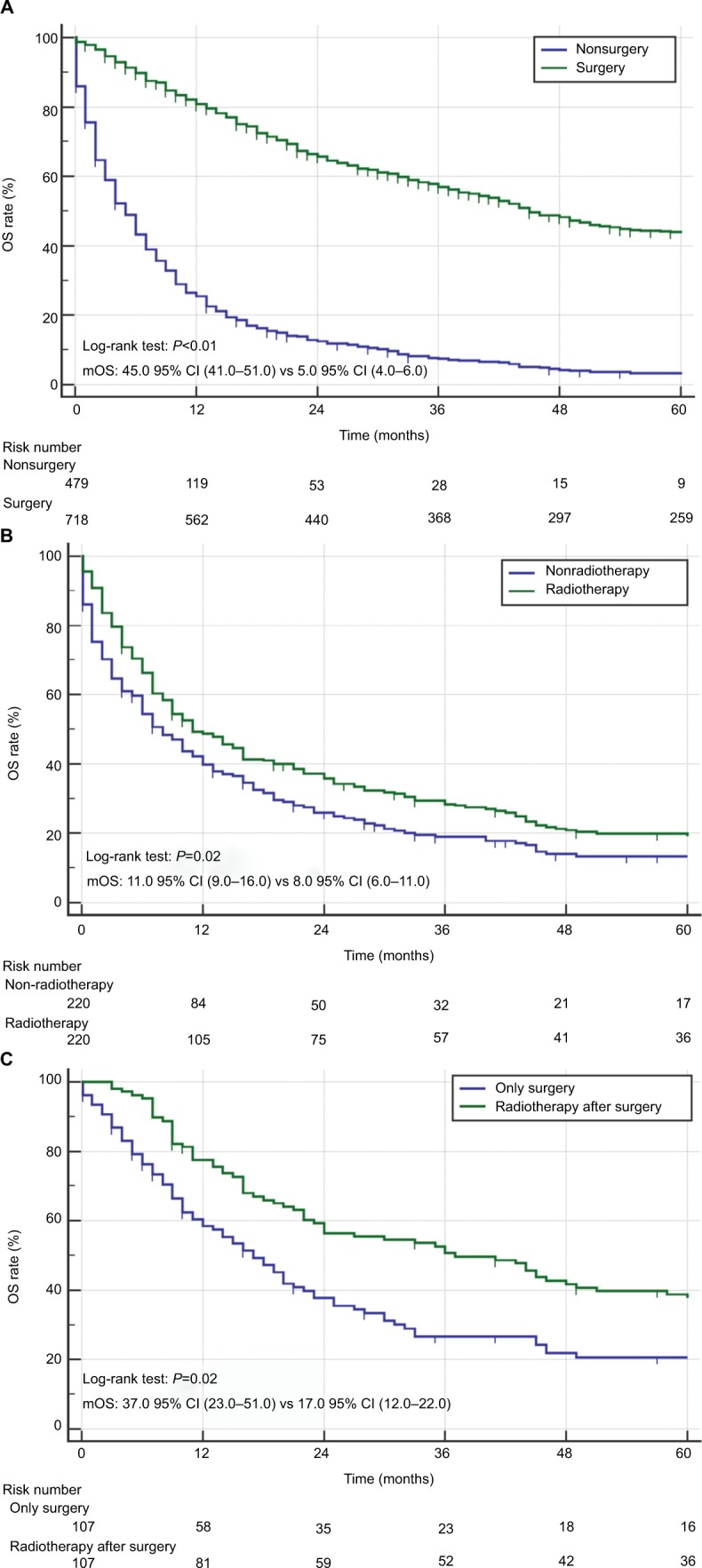
OS for CCAL patients treated with surgery and radiotherapy.
Notes: (A) OS for CCAL patients with/without surgery. (B) OS for CCAL patients with/without radiation. (C) OS for CCAL patients with radiotherapy after surgery or surgery only.
Abbreviations: CCAL, clear cell adenocarcinoma of the lung; mOS, median OS; OS, overall survival.
Table 2 also depicted variables that potentially influence OS. Older age, male, lesion of main bronchus, large tumor size, lymph node and distant metastasis, more advanced stages were associated with poor prognosis and surgical resection was significantly associated with prolonged OS (P<0.05 for all). Multivariate Cox analysis showed that being older than 65 years-of-age with larger or metastatic lesions were independent prognostic factors for worse OS (Table 3). Conversely, cancer-directed surgery was an independent protective factor, and this decreased the risk of death by more than half (HR =0.44, 95% CI 0.33–0.59).
Table 3.
Multivariate Cox proportional hazard analyses of clinical characteristics for overall survival rates in patients with CCAL
| Factor | Category | Multivariate | ||
|---|---|---|---|---|
| N | HR (95% CI) | P-value | ||
| Age (years) | <65 | 417 | Reference | |
| >65 | 421 | 1.54 (1.31–1.82) | <0.01 | |
| Gender | Female | 421 | Reference | |
| Male | 417 | 1.05 (0.89–1.22) | 0.59 | |
| Laterality | Left | 355 | Reference | |
| Right | 483 | 1.02 (0.87–1.20) | 0.38 | |
| Bilateral | 0 | – | ||
| Primary site | Main bronchus | 14 | Reference | |
| Upper lobe, lung | 526 | 1.42 (0.79–2.54) | 0.24 | |
| Middle lobe, lung | 48 | 1.97 (0.99–3.87) | 0.06 | |
| Lower lobe, lung | 232 | 1.58 (0.87–2.86) | 0.14 | |
| Overlapping | 18 | 1.35 (0.61–3.00) | 0.46 | |
| Tumor size (mm) | <39.9 | 498 | Reference | |
| >39.9 | 340 | 1.20 (1.02–1.42) | 0.03 | |
| Lymph node metastases | N0 | 510 | Reference | |
| N1 | 107 | 1.34 (1.04–1.73) | 0.03 | |
| N2 | 180 | 1.67 (1.33–2.11) | <0.01 | |
| N3 | 41 | 2.20 (1.49–3.25) | <0.01 | |
| Distant metastases | No | 691 | Reference | |
| Yes | 144 | 2.88 (2.20–3.78) | <0.01 | |
| Surgery | No | 190 | Reference | |
| Yes | 648 | 0.44 (0.33–0.59) | <0.01 | |
| Radiation | No | 608 | Reference | |
| Yes | 230 | 0.97 (0.79–1.20 | 0.81 | |
Abbreviation: CCAL, clear cell adenocarcinoma of the lung.
As shown in Figure S3, the optimal cut-points using X-tile program for age and tumor size were 66y, 73y and 22 mm, 52 mm, respectively. According to the optimal cut-points, enrolled CCAL patients could be divided into three groups. The Kaplan– Meier curve showed significant differences in OS among three groups for both age and tumor size (P<0.05 for all).
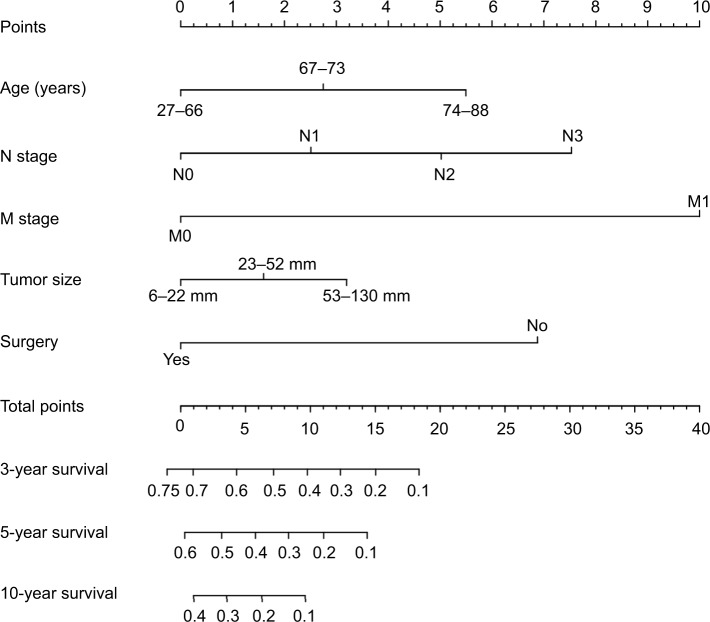
To predict the survival of CCAL patients, prognostic nomogram was established using all significant independent indicators for OS (Figure 3). The nomogram illustrated M category and N category as sharing the largest contribution to prognosis, followed by age and surgery. Tumor size showed a moderate impact on the survival. C-index for OS prediction was 0.72 (95% CI 0.69–0.74). The calibration plots for the probability of OS at 3, five or 10 year in CCAL patients cohort showed an optimal agreement between the prediction by nomogram and actual observation (Figure S4).
Figure 3.
Prognostic nomogram estimated by clinical characteristics for 3-, 5-, and 10-year OS in CCAL patients.
Notes: Each subtype within these variables could be assigned a score on the point scale. By summing the total score and locating it on the total point scale, a straight line was drawn down to determine the estimated probability of 3-, 5-, or 10-year survival.
Abbreviations: CCAL, clear cell adenocarcinoma of the lung; OS, overall survival.
Discussion
Most CCAL studies comprise a small series or single case reports due to the condition’s rarity. Therefore, clinicopatho-logical features and outcomes for this disease are unclear. Here, we describe the clinical characteristics of patients with CCAL and identified variables affecting OS using data from 1,203 patients from SEER database between 1988 and 2013. Further, a prognostic nomogram for CCAL patients was established and the predictive accuracy and discriminative ability of the nomogram were determined by a C-index and calibration curve.
From the previous sporadic cases, most CCAL patients were diagnosed at ~60 years-of-age. Approximately 28.8% of our CCAL patients were younger than this, and the age of diagnosis was 27–93 years, so our subjects were relatively older. Males were also slightly more prone to CCAL, and Whites were mainly affected. Most of CCAL patients had malignant lesions in the upper lobe, and Wang’s group found most lesions that occurred in the clear cell tumor of the lung (CCTL, a benign pulmonary tumor) were located in the lower lobes.9 Previous case reports also suggested that tumor size (diameter >2.5 cm) was associated with more aggressive CCTL.11,12 In the present study, most patients had larger tumor diameters than reported by Wang’s group.
Prognosis and treatment choices for CCAL may resemble those for common lung cancer, but the prognosis seemed to be better as indicated by median survival data that almost 15.6% and 5% patients could live more than 10, 20 years. In unmatched cohort, the mOS was 10 months in lung adenocarcinoma patients, while 19 months in CCAL patients. After PSM analysis, 787 CCAL patients were matched and the mOSs were 7 and 18 months, respectively. In matched cohort, CCAL was also an independent prognostic factor for poor prognosis in lung cancer patients when compared with lung adenocarcinoma. The prognosis seemed to be better in CCAL patients than those with lung adenocarcinoma, whatever in unmatched or matched cohorts. In CCAL patients cohort, the patients’ prognosis was significantly correlated with disease stages whatever TNM stage or SEER summary stage. For example, CCAL patients with TNM-I had a mOS of 63 months but only 4 months for patients with TNM-IV. Similarly, patients with larger lesions and metastases had shorter survival. Therefore, early detection may be required for optimal outcomes for CCAL patients. Similar to other types of lung cancer, older patients had worse survival outcomes.13 In univariate analysis, gender and primary site of tumor were correlated with the survival of CCAL, but after adjusting for other variables, no significant difference was noted.
Treatment regimens to achieve local and systemic control of tumor for CCAL patients also preserve the function and quality of life. Previous case reports showed that lesion resection should be a first line of therapy, if possible. If preoperative staging shows no evidence of metastases, a lobectomy and pneumonectomy are suggested, even for recurrent or metastatic patients. Li et al’s14 group reported that CCAL patients who received lobectomies after tumor recurrence were alive and tumor free after diagnosis. Chang et al’s15 group reported that CCAL patients who initially presented with multifocal bilateral choroid metastasis and received surgery had better OS. No significant difference in survival was observed among patients undergoing different types of surgery. If an early complete resection is performed, the 5-year survival for patients exceeds 43%. However, results from the Cox analysis show that only surgery decreased the risk of death and was an independent protective factor.
Adjuvant radiotherapy and chemotherapy are recommended for cases of incomplete resection, unresectable tumors, and patients with increased histological malignancy. However, the benefits for the prognosis of postsurgical chemotherapy or radiotherapy are currently being debated. Some reports suggest that CCAL patients have a low potential for metastasis and recurrence, so they do not require adjuvant chemotherapy after surgical treatment.9,14,16,17 However, a patient treated with operative chemotherapy had a marked recovery in the following 3 years after postoperative adjuvant chemotherapy and complete resection.18 In 2013, Chang et al’s15 group reported a case of CCAL with multifocal bilateral choroid metastasis that responded to systemic chemotherapy with cisplatin and pemetrexed after surgery. However, we lacked chemotherapy data for our study and could not assess this for CCAL patients. Radiation is reserved for patients with rare lung cancers, such as atypical carcinoid tumors, or for unresectable patients or those with serious comorbid conditions associated with greater mortality unrelated to the primary diagnosis.19,20 As we know, the present study is the first study to explore the clinical benefit of radiotherapy in CCAL patients. Overall, radiation did not benefit CCAL patients regarding OS, likely because CCAL patients with poor prognoses receive radiation treatment and not because radiation is ineffective. Conversely, we found that postoperative radiotherapy could prolong survival for 20 months compared with surgery alone. This was observed in CCAL patients with stages I–III and was statistically significant at the TNM-III stage.
To the best of our knowledge, this is the first nomogram for predicting the survival of CCAL patients that is based on SEER database with long-term follow-up. Both clinicians and patients could make an individualized survival prediction through this easy-to-use scoring system. Validation of the nomogram is essential to avoid overfitting of the model and determined generalizability.21 For common lung cancer, the validation of the nomogram often was based on the primary cohort and an independent validation cohort.22,23 Due to CCAL’s rarity, the present nomogram was only validated by the primary cohort. This is the biggest limitation for the present prognostic nomogram. Besides, the nomogram did not include comprehensive laboratory indices such as serum tumor markers and some important molecular factors (EGFR mutation, ALK fusion, and so on). Therefore, further efforts on prospective data collection and more clinical information are encouraged to improve this model.
Limitations
Similar to other studies using the SEER database, we had some limitations. First, the retrospective nature of this study and the inability to account for other relevant variables, such as performance status, were weaknesses. Although PSM analysis was performed, we still lacked a prospective study or a randomized controlled trial. Second, we lacked information about chemotherapy and could not account for the effect of potential advances in chemotherapy, thus limiting our ability to describe treatment patterns for CCAL patients. Third, responses to treatment and recurrence rates could not be ascertained from SEER. Finally, although calibration plots showed optimal agreement between predication and observation in the primary cohort, the repeatability and reliability of the established nomogram could not been guaranteed due to the lack of an independent validation cohort.
Conclusion
Despite the rarity of CCAL, we used a population-based approach to offer a crude stratification of prognoses based on commonly identified variables. CCAL patients have significantly longer OS compared to those with lung adeno-carcinoma. Older age, larger lesions, and lymph node and distant metastases were independent risk factors for CCAL, and cancer-directed surgery was an independent protective factor. Radiotherapy after surgery also prolonged survival. We established a novel nomogram for the prediction survival of CCAL patients. Physicians could estimate the survival probability of individual patient more precisely for this rare lung cancer. This is the largest series regarding clinical characteristics and outcomes for CCAL to date, so these data may be useful for future management and prospective studies in this patient population.
Supplementary Materials
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mcnamee CJ, Simpson RH, Pagliero KM, Meyns B, Hamilton-Wood C. Primary clear-cell carcinoma of the lung. Respir Med. 1993;87(6):471–473. doi: 10.1016/0954-6111(93)90076-c. [DOI] [PubMed] [Google Scholar]
- 2.Travis WBBA, Muller-Hermelinek HK. World Health Organization Classification of Tumours. Vol. 10. IARC Press; Lyon: 2004. [Google Scholar]
- 3.Liebow AA, Castleman B. Benign “clear cell tumors” of the lung. Am J Pathol. 1963;43:13–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards C, Carlile A. Clear cell carcinoma of the lung. J Clin Pathol. 1985;38(8):880–885. doi: 10.1136/jcp.38.8.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamato T, Yazawa T, Ogata T, Akaogi E, Mitsui K. Clear cell carcinoma of the lung: a case report and review of the literature. Lung Cancer. 1993;10(1–2):101–106. doi: 10.1016/0169-5002(93)90315-o. [DOI] [PubMed] [Google Scholar]
- 6.Iyoda A, Hiroshima K, Toyozaki T, et al. Clear cell adenocarcinoma with endobronchial polypoid growth. Pathol Int. 2000;50(12):979–983. doi: 10.1046/j.1440-1827.2000.01140.x. [DOI] [PubMed] [Google Scholar]
- 7.Kitada M, Ozawa K, Sato K, Hayashi S, Miyokawa N, Sasajima T. Clear cell carcinoma of the lung. Gen Thorac Cardiovasc Surg. 2010;58(2):87–90. doi: 10.1007/s11748-009-0471-8. [DOI] [PubMed] [Google Scholar]
- 8.Mizobuchi T, Masahiro N, Iwai N, Kohno H, Okada N, Nakada S. Clear cell tumor of the lung: surgical and immunohistochemical findings. Gen Thorac Cardiovasc Surg. 2010;58(5):243–247. doi: 10.1007/s11748-009-0513-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang GX, Zhang D, Diao XW, Wen L. Clear cell tumor of the lung: a case report and literature review. World J Surg Oncol. 2013;11(1):247. doi: 10.1186/1477-7819-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalkanis A, Trianti M, Psathakis K, et al. A clear cell tumor of the lung presenting as a rapidly growing coin lesion: is it really a benign tumor? Ann Thorac Surg. 2011;91(2):588–591. doi: 10.1016/j.athoracsur.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Kim WJ, Kim SR, Choe YH, et al. Clear cell “sugar” tumor of the lung: a well-enhanced mass with an early washout pattern on dynamic contrast-enhanced computed tomography. J Korean Med Sci. 2008;23(6):1121–1124. doi: 10.3346/jkms.2008.23.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaffey MJ, Mills SE, Askin FB, et al. Clear cell tumor of the lung. A clinicopathologic, immunohistochemical, and ultrastructural study of eight cases. Am J Surg Pathol. 1990;14(3):248–259. doi: 10.1097/00000478-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Zhang J, Shi F, Zhang C, Jiao Q, Zhu H. Better cancer specific survival in young small cell lung cancer patients especially with AJCC stage III. Oncotarget. 2017;8(21):34923–34934. doi: 10.18632/oncotarget.16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Zhu W, Khan RSU, Saeed U, Shi S, Luo Z. Primary clear cell carcinoma of the trachea: A CARE-compliant case report. Medicine. 2017;96(31):e7709. doi: 10.1097/MD.0000000000007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YS, Yeung L, Chang LC, Huang JS, Yeh KY. Choroid metastases revealing primary clear cell adenocarcinoma of the lung effectively treated with cisplatin and pemetrexed: a case report. J Med Case Rep. 2013;7(1):267. doi: 10.1186/1752-1947-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küng M, Landa JF, Lubin J. Benign clear cell tumor (“sugar tumor”) of the trachea. Cancer. 1984;54(3):517–519. doi: 10.1002/1097-0142(19840801)54:3<517::aid-cncr2820540322>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Santana AN, Nunes FS, Ho N, Takagaki TY. A rare cause of hemoptysis: benign sugar (clear) cell tumor of the lung. Eur J Cardiothorac Surg. 2004;25(4):652–654. doi: 10.1016/j.ejcts.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Shomura S, Suzuki H, Yada M, Kondo C. Surgically treated clear cell carcinoma of the lung; report of a case. Kyobu Geka. 2015;68(11):955–957. [PubMed] [Google Scholar]
- 19.Steuer CE, Behera M, Kim S, et al. Atypical carcinoid tumor of the lung: a surveillance, epidemiology, and end results database analysis. J Thorac Oncol. 2015;10(3):479–485. doi: 10.1097/JTO.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 20.He J, Shen J, Pan H, Huang J, Liang W, He J. Pulmonary lymphoepi-thelioma-like carcinoma: a surveillance, epidemiology, and end results database analysis. J Thorac Dis. 2015;7(12):2330–2338. doi: 10.3978/j.issn.2072-1439.2015.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 22.Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108(1):djv291. doi: 10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 23.Won YW, Joo J, Yun T, et al. A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer. 2015;88(2):201–207. doi: 10.1016/j.lungcan.2015.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.