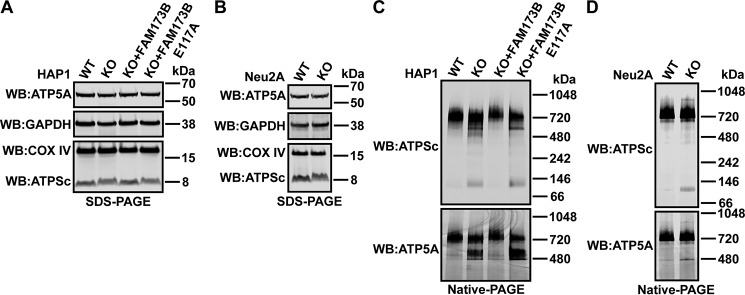
Figure 4.
Methylation at Lys-43 affects incorporation of ATPSc into the ATP synthase complex. A and B, denaturing gel (SDS-PAGE) and Western blot analysis of proteins present in cell extracts. A, unmodified HAP1 (WT), FAM173B KO, or KO cells complemented with FLAG-tagged FAM173B, either nonmutated or E117A-mutated, were lysed in 1% Triton X-100–containing lysis buffer. 50 μg of protein from lysates was resolved by SDS-PAGE and transferred by Western blotting (WB) to a membrane, which was probed with anti-COX IV antibody (loading control for mitochondria) and reprobed with anti-GAPDH antibody (loading control for cytosol). The same membrane was reprobed with anti-ATP5A and anti-ATPSc antibodies. Shown are images from a representative experiment. B, similar to A, but for lysates prepared from Neu2A-derived mouse cells. C and D, nondenaturing gel (native PAGE) and Western blot analysis of ATP synthase subunits present in mitoplast extracts. C, 4 μg of protein from mitoplast extracts prepared from HAP1-derived cells, as in A, was resolved by native PAGE and transferred by Western blotting to a membrane, which was probed with anti-ATPSc antibody and reprobed with anti-ATP5A antibody. Shown are images from a representative experiment. D, similar to C, but for mitoplast extracts prepared from Neu2A-derived mouse cells.