Abstract
Photosynthetic organisms often experience extreme light conditions that can cause hyper-reduction of the chloroplast electron transport chain, resulting in oxidative damage. Accumulating evidence suggests that mitochondrial respiration and chloroplast photosynthesis are coupled when cells are absorbing high levels of excitation energy. This coupling helps protect the cells from hyper-reduction of photosynthetic electron carriers and diminishes the production of reactive oxygen species (ROS). To examine this cooperative protection, here we characterized Chlamydomonas reinhardtii mutants lacking the mitochondrial alternative terminal respiratory oxidases, CrAOX1 and CrAOX2. Using fluorescent fusion proteins, we experimentally demonstrated that both enzymes localize to mitochondria. We also observed that the mutant strains were more sensitive than WT cells to high light under mixotrophic and photoautotrophic conditions, with the aox1 strain being more sensitive than aox2. Additionally, the lack of CrAOX1 increased ROS accumulation, especially in very high light, and damaged the photosynthetic machinery, ultimately resulting in cell death. These findings indicate that the Chlamydomonas AOX proteins can participate in acclimation of C. reinhardtii cells to excess absorbed light energy. They suggest that when photosynthetic electron carriers are highly reduced, a chloroplast–mitochondria coupling allows safe dissipation of photosynthetically derived electrons via the reduction of O2 through AOX (especially AOX1)-dependent mitochondrial respiration.
Keywords: algae, photosynthesis, electron transport system (ETS), chloroplast, mitochondrial respiratory chain complex, molecular imaging, respiration, reactive oxygen species (ROS), AOX, chloroplast-mitochondria, electron transport, high light, redox, oxidative stress
Introduction
Photosynthetic organisms convert absorbed light energy into chemical energy in the process of photosynthesis, which requires membrane-associated, light-dependent reaction centers, electron transport components, and ATP synthase; these components drive both ATP and NADPH formation, which in turn are required for the fixation of CO2 by the stromal localized Calvin–Benson–Bassham cycle (CBBC)3 (1). In the natural environment, photosynthetic organisms experience changes in light intensity and quality, temperature, nutrient levels, water availability and quality, and a variety of other factors. Fluctuating environmental conditions can impact metabolic processes, including photosynthetic electron transport (PET), and cause an imbalance between energy production and consumption, which in turn leads to over-reduction of the photosynthetic machinery that can result in oxidative stress and damage to various cellular components (2–4). To manage the absorption of excess excitation energy, photosynthetic organisms have evolved a diverse suite of mechanisms that tune energy production to consumption and dissipate excess absorbed light energy (2, 4–7). Many light-associated mechanisms characterized to date modulate photosynthetic outputs through nonphotochemical quenching, the activities of various alternative pathways for electron flow (3), and the rate of electron flow (8–10). Additionally, accumulating evidence indicates the importance of optimal mitochondrial respiration for sustaining photosynthetic activity (11, 12).
A functional link between chloroplasts and mitochondria has been demonstrated using inhibitors of mitochondrial respiration (13) as well as through characterizations of respiratory-deficient mutants (14–18). It was proposed that photosynthetically produced electrons, especially those generated during exposure of plants and algae to excess excitation energy, can be routed to mitochondrial respiration (reducing O2 plus H+ to H2O), which in turn can prevent hyper-reduction of redox components in the plastid and lessen the production of reactive oxygen species (ROS) and consequent cellular damage (11, 19). Moreover, for the diatom Phaeodactylum tricornutum, reductant from plastids can be delivered to mitochondria, where it is used for ATP production, which in turn can power various chloroplast processes (12). This energetic link between plastids and mitochondria involves the transfer of electrons (and ATP) between the organelles (e.g. chloroplasts to mitochondria) through the activities of metabolite shuttles, most notably the malate–oxaloacetate (5, 11, 19) and triose phosphate shuttles (20, 21).
An important characteristic of mitochondrial electron transport in plants, algae, some protists, and many fungi is the presence of an additional terminal respiratory oxidase called the alternative oxidase (AOX). AOX is a ubiquinol oxidoreductase that couples the oxidation of ubiquinol plus H+ with the reduction of O2 to produce H2O (22, 23). This oxidase is an interfacial membrane protein on the matrix side of the inner mitochondrial membrane that accepts electrons via the quinol pool from respiratory complex I or II, thereby bypassing the proton-pumping respiratory complexes III and IV. This respiratory activity uncouples electron flow from the development of a membrane potential, thereby reducing respiratory energy yield. Thus, mitochondrial electron transport is uncoupled from ATP production, and the free energy produced in electron transport is dissipated as heat (24, 25).
In vascular plants, functions attributed to AOXs are diverse and include the generation of heat, abiotic/biotic stress tolerance (26–32), and elimination of energized electrons when electron transfer components are highly reduced (i.e. serving as an electron valve), which prevents excess ROS production (33). AOX activity may help control ROS production by maintaining a favorable ratio of reduced to oxidized forms of ubiquinone, thereby preventing hyper-reduction of the respiratory electron transport chain (22, 23, 34). Control of the redox state of the ubiquinone pool can also be linked to maintenance of optimal energy metabolism, allowing the regeneration of oxidized cofactors and the continuous operation of glycolysis and the tricarboxylic acid (TCA) cycle. Overall, AOX appears to have a critical role in regulating aerobic redox balance under highly reducing conditions (35).
The unicellular green alga Chlamydomonas reinhardtii is a well-established genetic and physiological model organism that has been extensively used to examine various processes, including photosynthesis and acclimation to environmental change (3, 36–40). Mutants in genes encoding subunits of respiratory complexes in C. reinhardtii have been characterized for their photosynthetic performance under mixotrophic/heterotrophic conditions (14, 41). Diminished respiratory activity results in an elevated chloroplast redox state (more highly reduced plastoquinone pool), which in turn elicits a transition of the photosynthetic apparatus to state 2 (mobile light-harvesting antenna move from PSII to PSI) and an increased rate of cyclic electron flow (CEF) (42). Impairment of mitochondrial respiration and/or state transitions decreases the overall yield of photosynthesis and cell productivity (14, 43). The stm6 mutant of C. reinhardtii, which is disrupted for the MOC1 gene, which encodes a homologue of the mitochondrial transcription termination factor (mTERF), exhibits aberrant photosynthesis (44). Disruption of MOC1 causes a decrease in the level of mature transcripts encoding the nd1 subunit of the mitochondrial type-1 NADH dehydrogenase complex (complex I) (45). This mutant exhibits a reduced capacity for nonphotochemical quenching (46) and diminished growth under photoautotrophic, high-light conditions (17).
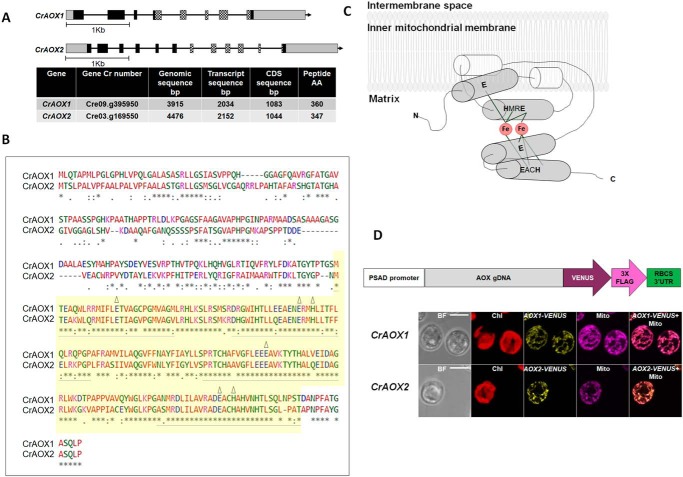
C. reinhardtii has two nuclear genes encoding mitochondria-localized alternative oxidases, CrAOX1 and CrAOX2 (Fig. 1A) (47, 48). The encoded proteins have high amino acid sequence similarity, especially at their C termini, and represent monomeric fungal-type AOXs (Fig. 1B) and not the homodimeric plant-type enzymes (24). These integral interfacial membrane proteins interact with the bilayer through two hydrophobic helices that are part of the nonheme di-iron carboxylate active site (49, 50) (Fig. 1C). The C-terminal hydrophilic domain of the protein has helices integral to the binuclear di-iron center, with two iron atoms ligated to four highly conserved glutamate/aspartate residues plus two histidine residues (Fig. 1C) (23, 48, 49, 51) (see “Results”).
Figure 1.
CrAOX1 and CrAOX2 genes and proteins and localization to mitochondria. A, characteristics of CrAOX1 and CrAOX2 genes and their encoded proteins. Top, CrAOX1 and CrAOX2 gene structures. Black boxes and black lines represent exons and introns, respectively, whereas checkered boxes correspond to the exons that encode the di-iron binding motif. The gray boxes are 5′- and 3′-UTRs. Bottom, table showing characteristics of the CrAOX genes and encoded proteins. B, pairwise alignment of CrAOX1 with CrAOX2 amino acid sequences (CLUSTAL 0(1.2.1) EBI (http://www.ebi.ac.uk/Tools/msa/clustalo/) (58)). (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.) The colors represent the physicochemical properties of the amino acid residues (noted at the bottom of the table). Yellow shading marks the conserved di-iron binding domain (82% identity). The four helices presented in the model of Andersson and Nordlund (51) are underlined in gray. The six conserved amino acid residues predicted to be ligands to the di-iron center (Glu, Glu, His, Glu, Glu, and His) are marked by triangles (23, 48). The amino acids are marked with colors: small + hydrophobic (red), acidic (blue), basic (magenta), hydroxyl + sulfhydryl + amine + G (green), and unusual (gray). C, model of the Chlamydomonas alternative oxidase structure on the inner mitochondrial membrane (51) as modified by Berthold et al. (64). Gray cylinders, four-helix bundle with the six conserved amino acids (in boldface type) predicted to be ligands to the di-iron (49). Red circles, bound iron atoms. Bonds are represented by light gray lines. N and C termini of the polypeptide are marked (adapted from Albury (63)). D, localization of CrAOX1 and CrAOX2 to mitochondria. Top, diagram of construct used to express the fusion proteins encoded by CrAOX1-VENUS and CrAOX2-VENUS. VENUS and the FLAG tag were fused at the CrAOX1/2 C terminus (pRam118; Fig. S1). White box, PSAD promoter; gray box, gDNA for AOX1 or AOX2 that extends from the ATG to the penultimate codon of the CDS; dark purple arrow, VENUS CDS; pink arrow, 3× FLAG sequence; green box, RBCS2 3′-UTR. Bottom, subcellular localization of CrAOX1 and CrAOX2 proteins in C. reinhardtii. Shown is expression of CrAOX1 (top) and CrAOX2 (bottom) VENUS fusion constructs. BF, bright field; Chl, chlorophyll autofluorescence; red, VENUS fluorescence; yellow, fluorescence from Mitotracker Red CMXRos (Mito); magenta, merged mitochondrial and VENUS signals (AOX1/2-VENUS + Mito). Bars, 5 μm.
The C. reinhardtii AOX genes are on different chromosomes, with CrAOX1 on chromosome 9 and CrAOX2 on chromosome 3. CrAOX1 is within a genomic cluster involved in nitrate assimilation (48) and is contiguous to NRT2;3, which encodes a nitrite/nitrate transporter (47). Similar to other genes in this cluster, CrAOX1 is induced by nitrate and repressed by ammonium (52), but it also responds to various stress conditions (25, 52–55). Until recently, CrAOX2 was thought to be constitutively or developmentally expressed, but at very low levels (52). Recently, this gene was shown to be induced (RNA level) by anoxia and copper deprivation (56).
As noted above, some studies have demonstrated that impaired mitochondrial activity perturbs C. reinhardtii's ability to efficiently acclimate to excess light and sustain functional photosynthetic activity under highly reducing conditions (14, 17, 43, 46, 57). CrAOX proteins were shown to be important for controlling stress responses and ROS levels (33), with CrAOX1 knockdown strains exhibiting hyper-reduction of the respiratory chain, which could trigger elevated ROS production. These cells also exhibited increased ROS scavenging, which decreased cellular damage, thereby increasing the cell's anabolic capacity (25). However, it is still unclear whether AOX proteins play a critical role(s) in allowing cells to adjust to the absorption of excess light.
In this study, we used a genetic approach to explore the roles of both CrAOX1 and CrAOX2 in light acclimation. Using VENUS-tagged proteins, we physically demonstrated that both AOX proteins are localized to mitochondria and characterized insertional mutants of both CrAOX1 and CrAOX2. These mutants exhibited greater sensitivity to very high light (HL; 1,200 μmol of photons m−2 s−1 unless otherwise indicated) than WT cells, although the phenotype of aox2 was not nearly as severe as that of aox1.
Results
CrAOX1 and CrAOX2 genomic sequences and protein domains
The genomic sequence of CrAOX1 (GenBankTM accession number AF314254 (Cre09.g395950.t1.2)) is 3,915 bp, contains eight exons and seven introns, and encodes a polypeptide of 360 amino acids with a molecular mass of 38.4 kDa. The genomic sequence of CrAOX2 (GenBankTM accession number AF314255 (Cre03.g169550.t1.1)) is 4,476 bp, contains 12 exons and 11 introns, and encodes a polypeptide of 347 amino acids with a molecular mass of 37.6 kDa (48) (Fig. 1A). Both predicted proteins contain a C-terminal ferritin-like di-iron carboxylate superfamily domain (Fig. 1B, shaded in yellow) that binds nonheme iron (Fig. 1C). This motif has homology to a dodecameric ferritin homologue that protects DNA from oxidative damage (59); rubrerythrin, which protects cells from toxic oxygen species (60); and di-iron carboxylate proteins that perform various redox reactions (61). This last category includes the AOXs (35). CrAOX1 also has a putative transcription activation Med15 domain at its N terminus (62). Pairwise alignment of the deduced CrAOX1 and CrAOX2 protein sequences (Fig. 1B) shows that they have 58% identity, with much higher identity (82%) over the conserved di-iron binding motifs that contain the six conserved amino acid ligands (Glu, Glu, His, Glu, Glu, and His marked by triangles in Fig. 1B, and liganded to iron in Fig. 1C) (23, 48, 51, 63, 64). AOX proteins also contain a conserved fold present in all other di-iron proteins and a hydrophobic crevice that has a ubiquinol-binding site (51) that leads from the membrane-binding region toward the di-iron center (23, 51, 64).
Recently, it was reported that the AOXs of Arabidopsis thaliana are differently post-translationally regulated by TCA cycle intermediates. This isoform-specific regulation was proposed to result in the noncompensated functions among AOXs (65). Furthermore, it was shown that post-transcriptional activation/inactivation of A. thaliana AOXs might be associated with conserved cysteine residues at the N terminus (66, 67). The A. thaliana AOXs have two highly conserved cysteine residues (CysI and CysII) present in the N-terminal domain of the protein that are involved in 2-oxo acid activation (66, 67). However, some other plant AOXs possess a serine residue instead of the cysteine at the CysI position (Fig. S1). These AOXs are insensitive to 2-oxo acid (e.g. pyruvate) activation, but instead may be activated by succinate under stress conditions (68–70). In addition to CysI and CysII, some plant AOXs possess a third conserved cysteine (CysIII) near the catalytic di-iron center that impacts the activity of the protein (67). Most AOXs contain a leucine residue at this position (LeuIII).
The alignments of CrAOX1 and CrAOX2 with other plant AOX isoforms (Fig. S1, A and B) show that the CrAOX2 possess the conserved CysI and LeuIII, whereas CrAOX1 has a serine instead of a cysteine at the CysI position. Furthermore, there is no Cys residue at the CysII position for either of the C. reinhardtii enzymes (Fig. S1). These differences in amino acids at key positions of the protein might indicate a distinct mode of post-transcriptional activation that may not involve disulfide bond formation.
CrAOX1 and CrAOX2 are in mitochondria
CrAOX1 and CrAOX2 have predicted presequences of ∼50 amino acids (48) that potentially form positively charged amphiphilic α-helices typical of mitochondria-targeting sequences (71). In silico predictions suggest that CrAOX1 is localized to mitochondria, whereas CrAOX2 is predicted to enter the secretory pathway (PredAlgo) (72). To experimentally determine the subcellular localization of these proteins, we fused the CrAOX genes (full-length genomic DNA) to the sequence encoding the fluorescent protein VENUS (Fig. 1D and Fig. S2), transformed the vector encoding the fusion constructs (Fig. S2) into WT C. reinhardtii, and screened transformants for VENUS fluorescence. For each fusion construct, we isolated three independent transformants with strong positive VENUS fluorescence signals (using a fluorescence plate reader) and then visualized the cellular locations of the signals by confocal laser-scanning microscopy. Both CrAOX1 and CrAOX2 localized to mitochondria based on congruence of VENUS and mitochondria-specific fluorescent signals (Mitotracker Red CMXRos) (Fig. 1D). No VENUS fluorescence was detected in transformants expressing Mitotracker Red marker in the absence of VENUS (same confocal settings) (data not shown).
Characterization of aox1 and aox2 mutants
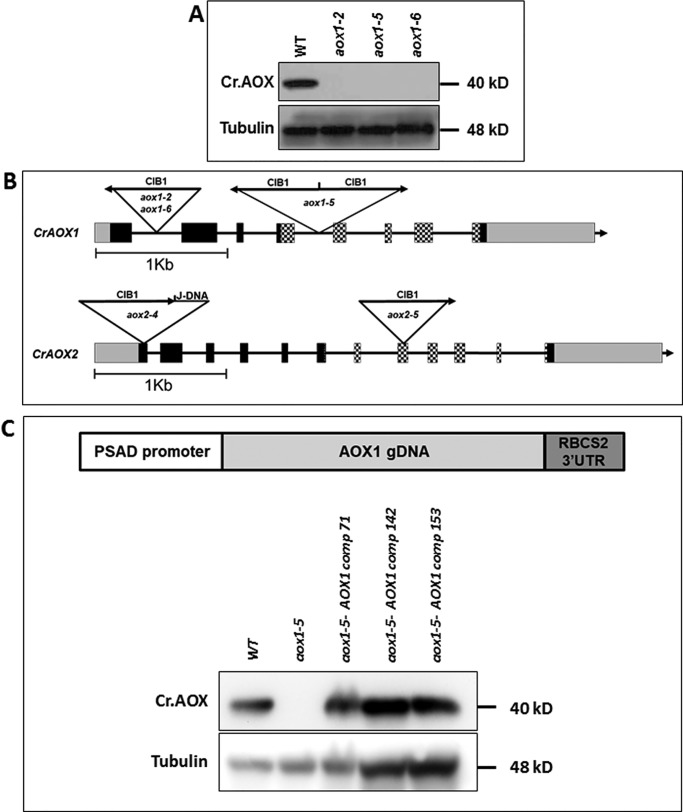
To explore AOX function in cells experiencing excess excitation, we obtained aox insertional mutants (insertion of cassette containing internal barcodes 1 (CIB)) from the CLiP mutant library (https://www.chlamylibrary.org/)4 (73). Initially, we selected eight putative mutants with insertions predicted to be in CrAOX1 and -6 with insertions in CrAOX2 (Table S1). To examine accumulation of CrAOX1 in these strains, we used a specific antibody (the antibody does not appear to react with AOX2) (5, 54); these antibodies show no signal in the mutant lacking AOX1 but do react to a specific protein in mutants disrupted for AOX2 (Fig. S3). Of the eight putative aox1 mutants, three appeared to be null for AOX1 (Fig. 2A and Fig. S4), whereas the others still synthesized the ∼36-kDa polypeptide detected with the AOX1 antibodies (Fig. S4, aox1-3 and aox1-4). The positions of the CIB insertions in the three null mutants, aox1-2, aox1-5, and aox1-6, were determined (73) (Fig. 2B); CIB was positioned within the first intron of aox1-2 and aox1-6, whereas aox1-5 harbored two CIB sequences inserted at the same position within intron 4, but in opposite orientations (this insertion splits the di-iron binding motif) (Fig. 2B).
Figure 2.
Insertions in aox mutants and their inability to synthesize AOX1 protein. A, immunoblots blot showing CrAOX1 protein in WT cells and the aox1-2, aox1-5, and aox1-6 mutants. AOX1 protein accumulation was assayed under mixotrophic, HL conditions. Tubulin was used as a loading control. Molecular masses of proteins are given to the right of the gel image. B, positions of CIB1 insertions in the CrAOX1 and CrAOX2 genes of aox1-2, aox1-5, aox1-6, aox2-4, and aox2-5 mutants. Arrows indicate the orientation of the insertions. The boxes and lines are defined in the legend to Fig. 1. C (top), general construct used to complement the aox1 mutant (pRam118-gAOX1-RBCS2 3′-UTR; see Fig. S6). Bottom, immunoblots showing CrAOX1 accumulation in WT cells (CMJ30), aox1, and three complemented strains (colonies 71, 142, and 153). AOX1 accumulation was assayed under mixotrophic, HL conditions. The tubulin protein was used as a loading control. Molecular masses of the proteins are given to the right of the gel image.
Because there are no available antibodies that recognize CrAOX2, we confirmed the CIB insertion sites in aox2-4 and aox2-5 (Fig. 2B) by PCR. Within the disrupted region of the aox2-4 strain, we identified a DNA fragment of about 1,054 bp from a different C. reinhardtii genomic region (noted as J-DNA, for “junk” DNA) that had inserted between the CIB and the genomic DNA, increasing the insertion size to 3,269 bp (Fig. S5, A and B). The aox2-4 mutant is likely null for CrAOX2 because the insertion disrupted the coding region of the gene at a site very close to the start codon. Furthermore, the insertion in aox2-4 resulted in a frameshift from amino acid 55, with a stop codon introduced immediately after amino acid 57, producing a truncated protein (Fig. S5C). We also confirmed the lack of CrAOX2 mRNA in both the aox2-4 and aox2-5 strains by RT-qPCR under conditions of dark anoxia. These conditions induce CrAOX2 according to transcriptomic data (phytozome) based on a recent publication (56) and our work (Fig. S5D). All subsequent experiments were conducted with aox1-5 and aox2-4.
Impact of AOX loss on fitness under HL exposure
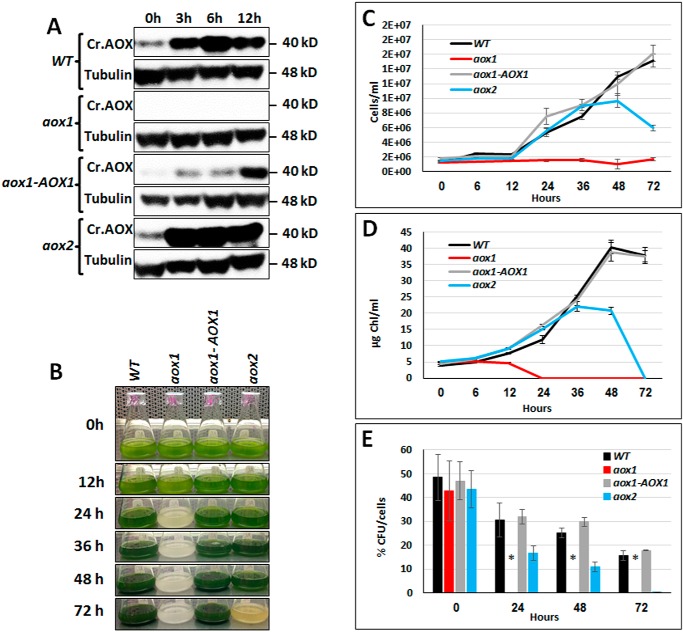
A number of studies have shown that C. reinhardtii cells impaired in mitochondrial activity exhibit reduced fitness and aberrant photosynthetic activity when they experience highly reducing conditions (14, 17, 43, 46, 57). Furthermore, mitochondrial CrAOX1 seems to diminish ROS accumulation when cells are exposed to high light (25). To elucidate the ways in which the two AOX isozymes impact cellular responses to HL, we cultured WT and mutant cells under mixotrophic conditions (Tris acetate phosphate (TAP)), which led to higher rates of mitochondrial respiration and elevated CrAOX transcript levels relative to photoautotrophic conditions (14). We quantified the levels of CrAOX1 protein in cells maintained in TAP medium in 60 μmol of photons m−2 s−1 (low light; LL) relative to those exposed to 1,200 μmol of photons m−2 s−1 (HL). The CrAOX1 protein in WT cells was detected in LL (Fig. 3A, 0 h) but accumulated to higher levels following HL exposure (Fig. 3A). To understand the role of the AOXs in managing excess absorbed light energy, we compared the growth and photosynthetic characteristics of the parental (WT), mutant, and rescued strains under HL conditions. The aox1 mutant was very sensitive to HL under mixotrophic conditions (Fig. 3, B–D); also shown is a combination of HL and high temperature (Fig. S6). To confirm that the loss of CrAOX1 was responsible for HL sensitivity, we introduced WT CrAOX1 expressed under the control of the PSAD promoter into the aox1 mutant background (Fig. S7 and Fig. 3B, aox1-AOX1); the growth characteristics of this strain were similar to those of the parental strain. Finally, like the AOX1-knockdown strain of Mathy et al. (25), our aox1 knockout line had a nearly 50% larger cell diameter during exponential growth under LL conditions than the parental and rescued strains (Fig. S8A). This finding may explain the increase in chlorophyll content in mutant cells (Fig. S8B).
Figure 3.
Accumulation of AOX1 protein in aox1 and aox2 mutants and their survival in high light. A, immunoblots showing CrAOX protein accumulation in C. reinhardtii WT, aox1, aox2, and the aox1-AOX1 rescued strain exposed to HL (1,200 μmol of photons m−2 s−1) following growth in 60 μmol of photons m−2 s−1. Samples were collected 3, 6, and 12 h after being exposed to HL, and the polypeptides in the samples resolved by SDS-PAGE and CrAOX1 were quantified by immunodetection using a monospecific antibody. CrAOX1 accumulation (top panels) was normalized to the level of tubulin (loading control); detection of tubulin was with a monospecific antibody. Molecular masses of the proteins are to the right of the image. B, image showing cultures of various strains (as indicated) in TAP medium, under HL conditions for various times, and up to 3 days (72 h). C, growth of the various strains (as indicated) in TAP medium, based on cell number, following exposure to HL for various times, and up to 3 days (72 h). D, chlorophyll levels in the cultures after exposure to HL for various times and up to 3 days. Data for the growth experiments (C and D) are based on three biological replicates (performed at the same time); error bars, S.D. Similar results were obtained in three independent experiments. E, survival (cfu/cell) of WT, aox1, the aox1-AOX1 rescued strains, and aox2. Samples were exposed to HL (1,200 μmol of photons m−2 s−1) for the indicated times (0–72 h) and were then plated onto solid TAP medium (1.5% agar plates) and grown in LL until colonies were observed (10 days). Student's t test was performed (*, significant difference (p < 0.05)). All results presented were derived from samples of cultures exposed to HL (1,200 μmol of photons m−2 s−1) for the indicated period.
The aox1 null mutant was very sensitive to HL compared with WT and the aox1-AOX1 and aox2 strains (Fig. 3, B–E). aox1 cells were unable to divide (Fig. 3C) and started to bleach after 12 h of exposure to HL (Fig. 3, B and D). After 24 h in HL, they appeared to be dead (Fig. 3E). The complemented strain recovered the ability to grow under these conditions, confirming the critical role of CrAOX1 in adaptation to HL. Interestingly, the aox2 mutant showed some sensitivity to HL, but the responses were much weaker and slower (Fig. 3, B–E). During the first 24–36 h of HL exposure, the aox2 mutant exhibited a rate of growth similar to that of WT cells based on both cell number (Fig. 3C) and chlorophyll concentration (Fig. 3D), although its growth and survival began to decline after 24–36 h, with a continued decline at later times.
The sensitivity to HL of WT, mutant, and rescued cell lines was also examined under photoautotrophic conditions (in Tris phosphate (TP) medium). We observed that the aox1 mutant exhibited greater HL sensitivity under photoautotrophic than under mixotrophic conditions. After 24 h at 1,200 μmol of photons m−2 s−1 in TP or TAP medium, the aox1 strain bleached and died, although the bleaching was more severe in TP (Fig. 3B and Fig. S9A). When the light intensity was lowered to 800 μmol of photons m−2 s−1, aox1 still showed greater HL sensitivity than WT cells, although it bleached more slowly. After 48 h, the aox1 mutant showed a clear difference from WT and appeared to be dead after 120 h (Fig. S9, B and C). Introduction of a WT copy of AOX1 expressed from the PSAD promoter rescued the aox1 phenotype. Interestingly, aox2 under photoautotrophic conditions behaved comparably with that of WT cells, which might indicate that CrAOX1 and CrAOX2 are important under different conditions, which is also reflected in the different ways in which they are regulated.
Compromised photosynthetic activity during exposure of aox1 to HL
Given that the phenotype of aox2 was not nearly as pronounced as that of aox1, we focused the remainder of our efforts on analyses of aox1 and the rescued strain during growth under mixotrophic conditions. To assess the importance of AOX1 in maintaining photosynthesis during exposure to HL, we monitored the dynamics of the quantum yield of PSII (F′v/F′m) for aox1, aox1-AOX1, and WT cells over the first 12 h of exposure to HL (Fig. 4A) (i.e. the first time point at which the bleaching phenotype was detected) (Fig. 3, B and D). The initial Fv/Fm values (t0, before exposure to HL) for all three strains were similar (WT, 0.65; aox1, 0.65; aox1-AOX1, 0.67). Interestingly, the aox1 mutant showed a dramatic reduction in Fv/Fm following a 3-h exposure to HL (0.29), whereas the WT and complemented strains retained F′v/F′m levels of 0.48 and 0.52, respectively. Furthermore, the F′v/F′m of the WT and complemented lines were similar throughout the time course of HL exposure; they retained an F′v/F′m of ∼0.4 (WT, 0.36; aox1-AOX1, 0.38) whereas the F′v/F′m of aox1 declined to near 0 after 12 h in HL (Fig. 4A). This analysis suggests that cells expressing normal levels of AOX1 are less susceptible to PSII photodamage and that they can exploit respiratory, AOX1-dependent electron transport as an “electron valve” that helps ameliorate the damage that can occur (through the accumulation of ROS and photoinhibition) when the cells experience hyper-reduction of PET.
Figure 4.
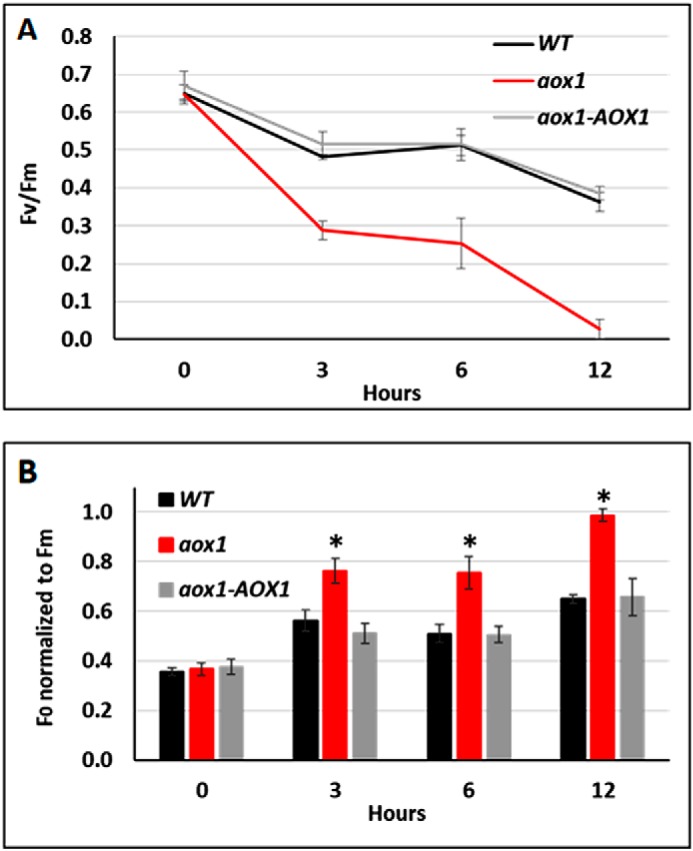
Functional analyses of PSII in WT, aox1 mutant, and the rescued strain. A, activity of PSII (F′v/F′m) at various times following the transfer of the cells to HL. Fv = Fm − F0. Fluorescence parameters were measured after a 30–60-min preincubation in the dark. The curves for WT, aox1-5 (aox1), and complement (aox1-AOX1) strains are distinguished by color as indicated. Data shown are means ± S.D. (error bars) (n = 4). B, time course of F′0 normalized to F′m. The bars for WT, aox1-5 (aox1), and the complement strain (aox1-AOX1) are distinguished by color as indicated. Data shown are means ± S.D. (n = 4). Student's t tests were performed, and the asterisk represents a significant difference (p < 0.05).
Hyper-reduction of PET frequently results in increased F0 values (relative to Fm), reflecting a reduction in the number of active PSII reaction centers (74). We therefore compared F0 levels (normalized by the Fm) in the three strains at different times following exposure to HL (Fig. 4B). Whereas the F0 values increased for all three strains following exposure to HL, the F0 of aox1 was always much higher than those of either the WT or complemented lines for all times tested following exposure of the cells to HL (3, 6, and 12 h). After 12 h in HL, the F0 for the aox1 strain was close to 1 (Fig. 4B), indicating that all reaction centers are damaged and do not function in charge separation, which is also reflected by the marked decline in the F′v/F′m (Fig. 4A). We therefore conclude that the decline in PSII quantum efficiency reflects highly inhibited PET, which is likely a consequence of an inability of the cells to adequately cope with high excitation energy.
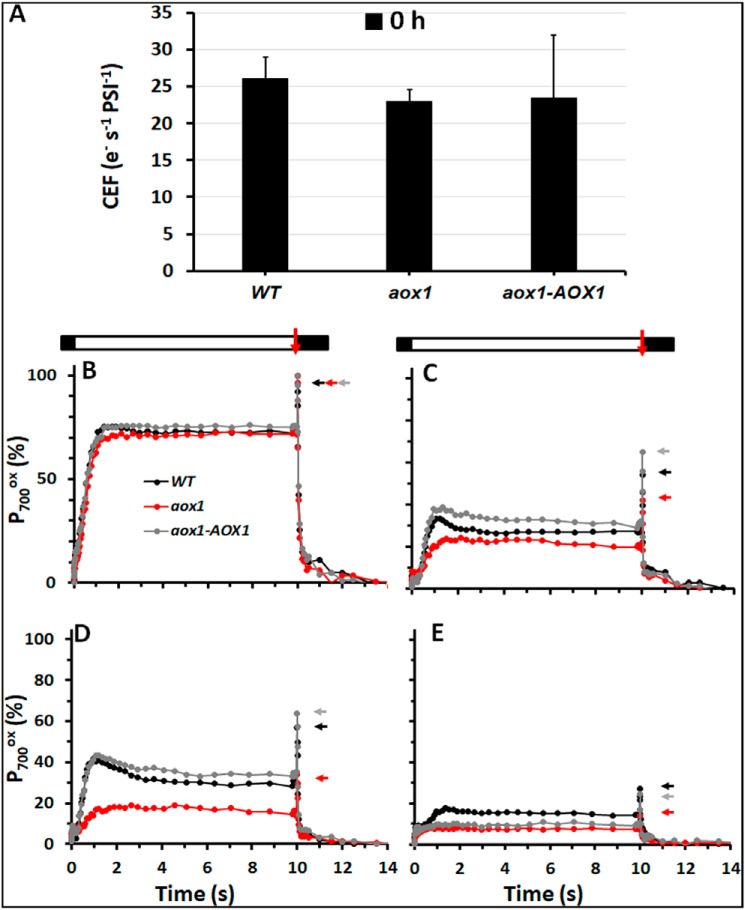
We also analyzed P700 oxidation/reduction kinetics to assess PSI activity following exposure of the cells to HL. Prior to HL treatment, all strains showed similar rates of CEF (26 e− s−1 PSI−1 for the WT and 23 e− s−1 PSI−1 for the mutant; Fig. 5A, 0 h). However, there was a strong impact of HL on PSI-related activity (Fig. 5, B–E). A 3-h exposure to HL caused a decrease in photooxidizable PSI to 54 and 65% (P700+ total − 0 h) for WT and the complemented strain, respectively, with a more pronounced decrease for the aox1 mutant, which had only 42% photooxidizable PSI. Whereas this values remain similar for the WT and complemented strains after 6 h of HL exposure, it decreased to 34% for the mutant. Finally, after 12 h in HL, all strains showed a precipitous decrease in PSI function that was accompanied by a decline in the level of PSI polypeptides (Fig. 6).
Figure 5.
Functional analyses of PSI in WT, aox1, and rescued strain. A, CEF rates under control conditions (0 h), for the various strains, as indicated. CEF measurements were also performed at 156 μmol of photons m−2 s−1 in the presence of 10 μm DCMU and 10 mm HA to inhibit any contribution from PSII, as described previously (106). The data are an average of four independent experiments. B–E, P700 kinetics in the WT, aox1-5 (aox1), and rescued (aox1-AOX1) strains over 12 h of HL treatment. PSI was oxidized and maintained at steady state in continuous light of 156 μmol of photons m−2 s−1 (white bar), followed by a saturating light pulse (red arrow from bar above the tracing) to generate total photooxidizable P700, which was then reduced during the dark period (black bar). PSI redox changes were measured at 705 nm and corrected by subtraction at 735 nm. 10 μm DCMU and 10 mm HA were added to the cells prior to the assay to inhibit PSII. The activity was measured at 0 h (B), 3 h (C), 6 h (D), 12 h (E). Colored arrows on the side of the tracing indicate the position of total photooxidizable PSI for each strain in each condition. The kinetics are the average of four independent experiments. Error bars, S.D.
Figure 6.
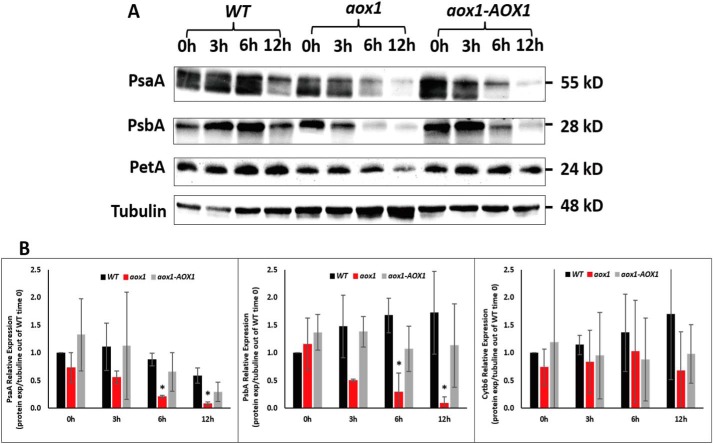
Levels of polypeptide subunits of PSI, PSII, and Cyt b6 in WT, aox1 mutant, and complement strains. A, immunoblots showing PsaA, PsbA, and PetA protein accumulation in C. reinhardtii cells of WT, aox1-5 (aox1), and complemented (aox1-AOX1) strains, resolved by SDS-PAGE on a 12% polyacrylamide gel. Antibodies used for the analysis were to the polypeptides indicated at the left. Protein extracts were loaded at a concentration of 2 μg of chlorophyll for each sample. See “Experimental procedures” for more details. Polypeptide subunits were as follows: PsaA for PSI, PsbA for PSII, and PetA for Cyt b6. B, quantification of PsaA, PsbA, and PetB protein levels. Bands were quantified by ImageJ software, and levels were normalized to the tubulin values and presented as relative to expression in WT cells at time 0. The data shown are the results of three biological replicates; error bars, S.D. Student's t test was performed (*, significant difference (p < 0.05)).
PSI and PSII abundance are reduced in aox1 mutants
To identify possible causes for the decreased photosynthetic activity in aox1 during HL exposure, we assessed the abundances of polypeptide subunits of the photosynthetic apparatus, including PsaA (PSI), PsbA (PSII), and PetA (Cyt b6f) following exposure of WT cells, the aox1 mutant, and the complemented strain to HL conditions. At the beginning of the experiment (time 0) the levels of PSI, PSII, and cytochrome b6f polypeptides were slightly lower (normalized to tubulin abundances) in the aox1 mutant compared with the WT and the complemented strains. After 3 h in HL, the levels of PsaA, PsbA, and PetA (normalized to tubulin) in all strains showed a minor reduction. The PsaA and PsbA levels were further depleted after 6 h of HL, with more severe depletion in the mutant relative to WT cells and the complemented strain (Fig. 6 and Table S2). After 12 h of HL, the PsaA and PsbA subunits were diminished in all strains, although the loss of these polypeptides was generally much more severe (normalized to tubulin abundances) in the mutant (Fig. 6B). The PetA level was also slightly lower in aox1 than in WT and the complemented strain following HL exposure, although this loss was not statistically significant. Overall, the loss of the photosynthetic activity, subunits of PSI and PSII, and the rate of bleaching were all more rapid in the aox1 mutant than in WT or the complemented strain.
HL causes elevated levels of singlet oxygen in the aox1 mutant
The formation of ROS in response to abiotic stress, such as HL, has been extensively characterized in plants (33, 52, 75, 76). Singlet oxygen (1O2) is a ROS that tends to accumulate to higher levels upon the absorption of excess light by chlorophyll, predominantly in light-harvesting complexes and PSII reaction centers. The 1O2 molecule readily oxidizes many biological molecules and therefore is toxic to cells (77–79). It has been suggested that AOX activity can reduce ROS formation by removing electrons from the ubiquinone pool (80). To understand the causes of HL sensitivity and damage to the photosynthetic apparatus in the aox1 mutant, we assessed in vivo accumulation of 1O2 in WT, aox1, and aox1-AOX1 strains using the fluorescent chemical probe Sensor Green. Cell cultures were aliquoted into 96-well plates and exposed for 30 min to either dark, LL, or HL, and the fluorescent signals from the probe were monitored using a microplate fluorescence reader (Infinite M1000, TECAN). HL treatment elicited increased levels of fluorescence from the probe in all strains compared with dark or LL controls, although the aox1 mutant showed the most pronounced increase in fluorescence in HL but also showed a high fluorescence signal in LL relative to the other strains (Fig. 7).
Figure 7.
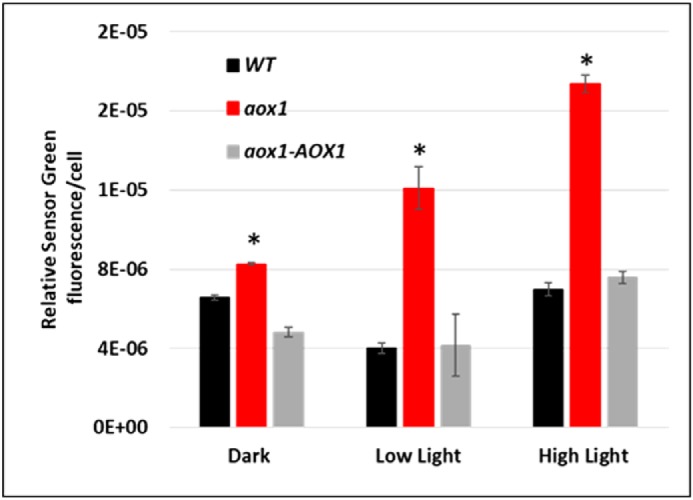
ROS accumulation in WT, aox1, and the rescued strain. Cultures of WT, aox1-5 (aox1), and the rescued strain (aox1-AOX1) were grown in TAP medium in 60 μmol of photons m−2 s−1 to exponential stage (1–2 × 106 cells m−1) and then incubated in the dark for 15 min in medium containing a final concentration of 10 μm fluorescent probe Sensor Green, which detects singlet oxygen (1O2). Cells were washed with TAP medium, and cells containing 100 μg of chlorophyll were aliquoted into individual wells of 96-well microtiter plates. The microtiter plates were maintained either in the dark, in 60 μmol of photons m−2 s−1, or in HL for 30 min. The fluorescent signal of the probe was monitored using a fluorescent microplate reader (Infinite M1000, TECAN). The data shown are the results of four biological replicates; error bars, S.D. Student's t test was performed (*, significant difference (p < 0.05)).
Discussion
Evidence has been accumulating that suggests a role for mitochondrial electron transport in the maintenance of photosynthetic activity under stress conditions (5, 12). Cooperation of plastid photosynthesis and mitochondrial respiration has been reported for vascular plants (11, 16, 19, 81–83) and microalgae (5, 12, 18, 57, 84). However, the importance of AOXs for acclimation of photosynthetic organisms to excess absorbed light energy has still not been clearly detailed.
In the present study, we examined potential roles of AOX in the acclimation of C. reinhardtii to excess absorbed light energy by characterizing null mutants for the CrAOX1 and CrAOX2 genes. The AOX proteins were localized to mitochondria. Mutants devoid of AOX1 produced more ROS than WT cells or the complemented strain and were HL-sensitive; the aox1 strain died much more rapidly following transfer to HL than aox2. Therefore, CrAOX1 likely helps control the ratio of reduced to oxidized forms of ubiquinone in mitochondria, and mitochondrial redox balancing appears to be coupled to plastid redox poise, which has a crucial role in protecting the photosynthetic machinery from hyper-reduction, oxidative damage, and HL-induced cell death.
Elimination of AOX and high-light fitness
When grown under HL conditions, WT cells, the complemented strain and the aox1 mutants all exhibited depressed photosynthetic activity (Figs. 4 and 5), a loss of polypeptides associated with PSI and PSII (Fig. 6), and elevated ROS accumulation (Fig. 7). However, much more ROS was produced by aox1 than WT cells and the complemented strain. Furthermore, whereas WT cells grew and remained viable under the HL conditions used, the aox1 mutant bleached and died after being exposed to HL for 24 h (Fig. 3, B–E). Measurements of photosynthetic activities have suggested that the HL treatment of aox1 caused saturation of both plastid and mitochondrial electron transport (2, 4, 6, 7, 9, 38) more rapidly than for WT and the complemented strain, and, based on this study, additional mechanisms responsible for dissipating excess absorbed energy were not able to fully compensate for the loss of CrAOX1.
In plants, a number of studies have focused on the energy-dissipating role of AOX and reported its involvement in HL responses (85, 86). In Arabidopsis thaliana, aox mutants were more sensitive than WT plants exposed to a combination of HL and drought conditions (31). Furthermore, work with Nicotiana tabacum demonstrated the involvement of AOX in preventing hyper-reduction of the chloroplast electron transport chain and photodamage under drought conditions (87). Studies with C. reinhardtii have shown that AOX is part of the cellular acclimation response to the absorption of excess light energy. One study assessed functional and proteomic characteristics of an AOX1 silenced strain (25). Cells with reduced levels of AOX1 exhibited hyper-reduction of the respiratory chain and elevated production of ROS relative to WT cells following transfer of the cells from LL to moderate/HL conditions (from 50 to 200 μmol of photons m−2 s−1 in this case). The authors concluded that the mutant cells acclimated to the moderate/HL conditions by increasing ROS scavenging, decreasing activities that would generate reducing power and elevating the anabolic reaction capacity, all processes that would diminish accumulation of reducing power (25). Although some conclusions of our study and the one of Mathy et al. (25) are similar, the HL in our system was much higher (but still physiological), which led to severe damage to the photosynthetic apparatus (Figs. 4–6), elevated ROS levels (Fig. 7), and, even after relatively short times, a more pronounced death of aox1 than of either WT or the complemented strain; the aox2 mutant was not nearly as sensitive to HL as the aox1 strain.
Dang et al. (5), showed that a C. reinhardtii mutant lacking the PGRL1 protein (pgrl; PGRL is associated with CEF) had elevated AOX activity and CrAOX1 protein accumulation under photoheterotrophic conditions in HL and ambient CO2 conditions (5). These results suggest activation of AOX-dependent, mitochondrial dissipation of reducing power as part of the energy-dissipating strategy that allows the cells to manage excess reductant under conditions in which PET becomes hyper-reduced.
Elimination of AOX impacts photosynthesis under high-light conditions
In this study, we show that the absence of CrAOX1 in C. reinhardtii resulted in severe damage to PSII and PSI (Figs. 4–6), even after as little as 3 h in HL. HL-exposed aox1 had an elevated F′0/F′m (Fig. 4B) relative to WT and the complemented strain, with a diminished capacity to use photosynthetically generated electrons. A 3-h exposure to HL caused a marked decrease in both PSI and PSII activity for all strains. The total fraction of P700 oxidized was significantly lower in the mutant than in the WT and the complement strains. At this 3-h HL time point, there is a only a small decrease in the level of PSI and PSII polypeptide subunits for all of the strains (Fig. 6), suggesting that the diminished PET observed after a relatively short exposure to HL mostly represents dysfunction rather than loss of reaction centers. The apparent loss of PSI activity could reflect the tendency for HL-exposed cells (especially the aox1 mutant) to be limited for PSI acceptor molecules, which would promote charge recombination (88). AOX knockdown plants under severe drought conditions exhibited an increase in their redox state (87, 89) that triggered charge recombination, which diminished the fraction of photooxidizable PSI reaction centers. Whereas all of the strains may be experiencing charge recombination, the fraction of photooxidizable P700 is most diminished in aox1.
Energetic interactions between chloroplast and mitochondria
Our data indicate that CrAOX1 in mitochondria could dissipate excess energy caused by the absorbance of HL, providing evidence for cross-talk between chloroplasts and mitochondria. Electrons from PSII are used in the production of ATP and NADPH. In chloroplasts, ATP and NADPH can be used in the CBBC to reduce carbon dioxide to triose phosphate (triose-P), whereas reductant (NADPH) can also be used to synthesize malate (MAL) from oxaloacetate (OAA). Plants and algae have a MAL-OAA shuttle that can move electrons between chloroplasts and mitochondria (11, 90). A key enzyme for the operation of the shuttle is malate dehydrogenase (MDH), which catalyzes the interconversion of MAL and OAA coupled to the oxidation/reduction of NAD(H)/NADP(H). Vascular plants and C. reinhardtii contain multiple MDHs that have been detected in chloroplasts, mitochondria, the cytosol, and peroxisomes (21, 90). The MAL/OAA translocator, another important component of the shuttle, is involved in transporting MAL and OAA across the envelope membranes of organelles. Additionally, triose-P could be exported from chloroplasts to the cytosol via triose phosphate–phosphate transporters. Once in the cytosol, triose-P could be converted to acetyl-CoA, which is a substrate of the TCA cycle. Hence, the shuttles appear to facilitate trafficking of metabolites and electrons among the different organelles, which may be reflected by an increase in ETR and reducing power observed when cytosolic MDH was overexpressed in apple cells (91). In addition to the shuttling of reducing equivalents, chloroplast ATP antiporters and mitochondria ATP carriers have been characterized in vascular plants (92, 93) and identified in C. reinhardtii (21). Interestingly, upon exposure of C. reinhardtii to HL, metabolites associated with both the CBBC and TCA cycle increased, suggesting rapid movement of metabolites from chloroplasts to mitochondria. Rapid exchange of metabolites between mitochondria and chloroplasts may be facilitated through direct physical associations between the organelles; it was noted that mitochondria are centrally positioned in C. reinhardtii cells under mixotrophic, low-light conditions, whereas upon exposure to high light, they move to the cell's periphery, becoming wedged between the plasma membrane and chloroplast, where they appear appressed to the chloroplast envelope (94).
Our results are in accord with several plant and algal studies focused on the role of AOX in modulating PET and photodamage in HL. In A. thaliana, AOX appears to be important for moderating changes in the redox state of ubiquinone and plastoquinone pools upon HL exposure (85, 95). Moreover, the capacity of the AOX system appears to increase in CEF-PSI mutants under HL conditions (86, 96), whereas a stm6 mutant of C. reinhardtii was unable to fine-tune mitochondrial OXPHOS complex I (NADH dehydrogenase) and exhibited enhanced respiration through AOX and compromised chlororespiration and CEF, likely a result of the more oxidized state of the chloroplast stroma (57). Studies described above as well as our results demonstrate the critical integration of AOX activity into photosynthetic electron flow and sustaining cell viability under conditions resulting in hyper-reduction of the photosynthetic apparatus and a greater tendency to generate ROS. Our work unequivocally demonstrates the physical occurrence of CrAOX1 and CrAOX2 in mitochondria, that the aox mutants are more sensitive to HL than the complemented strain (with the aox1 null strain more light-sensitive than the aox2 null strain), that light provokes high levels of ROS in aox1, and that the two AOX polypeptides in C. reinhardtii appear to function under different conditions.
Experimental procedures
Growth conditions and strains
Cultures were maintained on solid (1.5% agar (w/v)) TAP medium (pH 7.2) at 25 °C and 30 μmol of photons m−2 s−1 (cool white fluorescent lights; F34CW/RS/WM/ECO; Ecolux) using a modified trace element solution (97). Cells were grown in 90 ml of liquid TAP medium in 250-ml Erlenmeyer flasks at 25 °C, under continuous cool white fluorescent lights (F34CW/RS/WM/ECO; Ecolux) of 60 μmol of photons m−2 s−1 and were shaken at 170 rpm. Cells used for experiments were grown under the same conditions but with continuous light and full-PAR LEDs (from −700 nm, diodes: 660-nm red, 55.4%; white, 21.4%; 440-nm blue, 23.2%). LL was 60 μmol of photons m−2 s−1, whereas HL was 1,200 μmol of photons m−2 s−1. Cells were also grown in TP medium.
All mutant strains for these studies (Table S1) and the parental CMJ30 (CC-4533 cw15 mt-) reference strain can be obtained from the Chlamydomonas Resource Center (73) (https://www.chlamycollection.org/). Genomic characterizations of the insertional mutations were performed using the PCR-based method described previously (73), with primers given in Fig. S1.
Transformation of C. reinhardtii
Transformation was performed by electroporation (Zhang 2014) using the GenePulser II Electroporator (Bio-Rad). The reactions contained 200 ng of linearized plasmid mixed with 250 μl of 2 × 108 C. reinhardtii cells ml−1. The electroporation parameters were 800 V (2 kV cm−1) and 25-microfarad capacitance using a standard 0.4-cm cuvette (98, 99). Transformed C. reinhardtii cells were selected on TAP agar medium containing 20 μg ml−1 hygromycin in 5–10 μmol of photons m−2 s−1. After the colonies grew, they were selected for further analyses.
Subcellular localization of AOXs
The pRam118 plasmid was provided by Dr. Silvia Ramundo. This plasmid was constructed by replacing the aphVIII paromomycin resistance gene with the aphVII hygromycin resistance gene from plasmid pLM005 (100). Assembly of the pRam118-AOX-VENUS plasmids (Fig. S2) was performed by Gibson assembly (101).
Cells were transformed with the pRam118-AOX-VENUS plasmid, and ∼200 potential transformants were transferred to individual wells of a 96-well, flat-bottom microtiter plate (Greiner Bio-One 655101) with each well containing 150 μl of TAP medium, 5 μg ml−1 hygromycin. Cultures in these plates were grown for 3 days with shaking at 200 rpm under 30 μmol of photons m−2 s−1 cool white fluorescent light, refreshed by adding 75 μl of fresh medium (∼50% of the volume remaining in the well) and grown for an additional day. Transformants were then screened for VENUS expression using a fluorescent microplate reader (Infinite M1000, TECAN). Parameters used were as follows: VENUS (excitation 515/12 nm and emission 550/12 nm) and chlorophyll (excitation 440/9 nm and emission 680/20 nm). The VENUS fluorescence signal was by normalization to the chlorophyll fluorescence signal (102). For each gene, we selected three independent transformants that showed a minimum of 4× VENUS:chlorophyll fluorescence and grew them to a concentration of 2–4 × 106 cells ml−1. The signal of the transformed lines was visualized using a TCS SP8 confocal laser-scanning microscope (Leica). For co-localization of the VENUS signal and a cellular compartment, we visualized chloroplast and mitochondria using autofluorescence and Mitotracker Red CMXRos (Thermo Fisher Scientific), respectively. For the latter, cultures were incubated for 10 min with MitoTracker at a concentration of 1 μm and then spotted onto a polylysine-coated slide for imaging. The imaging conditions/settings used were as follows: cells maintained at room temperature and imaged in resonant scanning mode using LASX software and a ×63, numerical aperture 1.4 oil objective, with 16-line averaging and 2-frame accumulation. Excitation/emission settings were 514-nm/525–550-nm HyD SMD hybrid detector for Venus, 561-nm/582–617-nm PMT detector for Mitotracker Red, and 561-nm/650–700-nm HyD SMD hybrid detector for chlorophyll autofluorescence. The VENUS signal was collected sequentially and separately from those of Mitotracker Red and chlorophyll to maximize the specificity of detection. To visualize VENUS, a lifetime gate filter (1–6 ns) was used to reduce background resulting from chlorophyll autofluorescence. Z-stacks of ∼20 slices that were 0.2 μm apart were obtained for each field of view. Images shown (projections of signals obtained from 4–6 slices) were analyzed using Fiji software (109) and collected from a minimum of 10 independent biological replicates for each strain.
Complementation of AOX1
Complemented lines were generated using the full-length CrAOX1 gDNA from the first codon (ATG) to the stop codon. The gDNA was used in a Gibson assembly to generate pRam118-gAOX1-RBCS2 3′-UTR (Fig. S7 and Fig. 2C). In this construct, CrAOX1 is under the control of the PSAD promoter and the RBCS2 3′-UTR. This construct was transformed into the aox1 mutant using electroporation (described above). A large number of hygromycin-resistant colonies (∼200) were selected and streaked onto solid agar medium containing 20 μg ml−1 hygromycin, and colony PCR was used to confirm the site of the insertion; both sides of the AOX1 gDNA, the PSAD promoter and RBCS2 3′-UTR (Fig. S7), were amplified using two sets of primers: primer set 1, PSAD promoter FP (5′-AGGTTTCCTCGCCGAGCAAG-3′) and gDNA-AOX1 RP (5′-TCTTCTGGGCTCCATAGCT-3′); primer set 2, gDNA-AOX1 FP (5′-CTCAAGCCCGGTGCCAA-3′) and RBCS2 3′-UTR RP (5′-GGAGCGGGGATCCTTAACGCTTCAAATACGCCCAGC-3′). Colonies in which both sides of the insertion (∼25%) were detected were grown in liquid medium to 2–4 × 106 cells ml−1, exposed to HL for 4 h, and then harvested for protein extraction and immunoblotting. Restoration of AOX1 protein accumulation in the transformed aox1 mutants was demonstrated for three transformants based on reactivity with the anti-AOX1 antibodies, which were purchased from Agrisera (54) (Fig. 2, A and C).
SDS-PAGE and immunoblot analysis
SDS-PAGE and immunoblot analyses were performed as described previously (103). C. reinhardtii cells were grown in liquid cultures to 2–5 × 106 cells ml−1 and exposed to HL as described under “Growth conditions and strains.” 1–2 × 107 cells ml−1 were collected by centrifugation (1,200 × g, 4 min), made homogeneous in resuspension buffer (5 mm HEPES (pH 7.5, KOH), 10 mm EDTA (pH 7.5, NaOH), 100 mm DTT, 1 mm phenylmethylsulfonyl fluoride, 1 mm ϵ-amino-n-caproic acid, and 1 mm benzamidine HCl), collected again by centrifugation (10,000 × g, 1 min), and then resuspended in extraction buffer (resuspension buffer supplemented with 100 mm Na2CO3, 100 mm DTT, 2% (w/v) SDS, and 12% (w/v) sucrose). Samples were boiled for 1 min, briefly cooled on ice, and clarified by centrifugation at 21,000 × g for 2 min. The solubilized polypeptides were resolved on a 10–12% polyacrylamide gel (Bio-Rad) by SDS-PAGE (30 min at 60 V, 60 min at 120 V), and the resolved proteins were transferred from the gel to polyvinylidene difluoride membranes using the Pierce Power semi-dry blotter system (Thermo Fisher Scientific). Blotted polyvinylidene difluoride membranes were blocked with a 5% (w/v) suspension of powdered milk in TBS with 0.1% (w/v) Tween 20 (TBST) before a 1-h incubation (shaking on a rocking table) at room temperature in the presence of primary antibodies. All primary antibodies were from Agrisera except for that of α-tubulin, which was from Sigma (T5168), and were used at dilutions recommended by the manufacturer. The membranes were then washed three times for 10 min each with TBST and incubated with TBST 3% powdered milk (w/v) containing the secondary antibodies, horseradish peroxidase–conjugated anti-rabbit IgG (Life Technologies, 656120), or goat anti-mouse IgG (Life Technologies, G2104). After the membranes were washed three times for 5 min each with TBST, peroxidase activity was detected by chemiluminescence (Advansta).
Chlorophyll concentration and cell count
C. reinhardtii cells were collected in 1.5-ml tubes by centrifugation (21,000 × g, 5 min), and chlorophyll concentrations were determined after extraction by methanol (104). Cell number and size were determined using the Countess II automated cell counter (Thermo Fisher Scientific). Samples were analyzed in triplicate.
Spectrophotometric measurements
Cells were collected by centrifugation at 2,500 × g for 5 min at room temperature, and cell pellets were resuspended in TAP + 10% Ficoll (w/v) to prevent cell settling. The cells were diluted to a final concentration of ∼6 × 106 ml−1 and placed into 50-ml Erlenmeyer flasks, where they were shaken at 340 rpm for 30 min in the dark. The JTS-10 spectrophotometer (Bio-Logic, Grenoble, France) was used to measure absorption changes for each strain. Detection and actinic lights were provided by LEDs, and the saturating flash was provided by a dye laser (640 nm). The detection wavelength was selected using interference filters, 520 nm to measure the electrochromic shift signal upon the onset of continuous light. Changes in the oxidation state of P700 were monitored at 705 nm, with a correction based on absorbance at 735 nm (which eliminates the nonspecific contribution at 705 nm). Blue (BG-39, Schott) and red (RG 695) filters were used to eliminate excitation light from the measurement. The ratio between active PSI and PSII centers was estimated using the amplitude of the fast phase (1-ms a phase) of the electrochromic shift (ECS) (520-nm) signal upon excitation with a laser flash, in the presence and absence of 10 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) plus 1 mm hydroxylamine (HA), which inhibit PSII activity. PSII values were calculated from reduction in the amplitude of the fast phase following the addition of the inhibitors (105). The PSI value was obtained from the amplitude of phase a after DCMU + HA additions. The ratio was normalized to the PSII active centers (Table S2). The CEF rate was estimated as described previously (106).
Chlorophyll fluorescence analysis
Chlorophyll fluorescence measurements were performed as described previously (10). Cells were centrifuged (1,200 × g, 5 min), and the pellet was resuspended to a concentration of 10 mg ml−1 chlorophyll in TAP medium. Fluorescence measurements were performed using a Walz Dual-PAM-100 fluorometer on the “light curve” setting or on the single saturating pulse setting. Prior to the assay, cells were acclimated to the dark for at least 30 min and then exposed to a saturating light pulse, which enabled calculations of Fv/Fm and F0/Fm. The PSII yield was derived from the equation, ΦPSII = (Fv/Fmax) where Fv = Fm − F0 (107).
ROS measurements
In vivo production of 1O2 was evaluated using Sensor Green reagent (1O2 Sensor Green, Life Technologies, Inc., S36002) fluorescent probes. Cells from exponentially growing cultures were incubated with the probes in the dark for 15 min, at a final concentration of 10 μm. Cultures were then washed with TAP medium, and 100-μl aliquots were placed in wells of 96-well microtiter plates, where they were maintained either in the dark, LL, or HL for 30 min. The fluorescent signals of all probes were collected using a fluorescence microplate reader (Infinite M1000, TECAN). Parameters used were as follows: for probe, excitation 480/20 nm half-bandwidth and emission 530/16 nm half-bandwidth; for chlorophyll, excitation 440/9 nm half-bandwidth and emission 680/20 nm half-bandwidth. The fluorescence signal was determined by subtracting basal autofluorescence of control cells from the signal of the cells containing the dye. The signals were normalized to the number of cells in each sample.
RT-qPCR
RT-qPCR was performed as described previously (108). Briefly, total RNA was isolated using the RNeasy plant minikit (Qiagen) and treated with DNase I (Qiagen). First-strand cDNA was generated by reverse transcription of 0.5 μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR using the Roche Light Cycler 480 was performed with the SensiMix NO-Rox SYBR Green I kit as described by the manufacturer (Bioline). Samples generated by first-strand cDNA synthesis were used as a template in PCRs of 20 μl, with 400 nm forward and reverse primers for detection of CrAOX2: FP, 5′-TGATCTTCCTGGAGACCATTGCTG-3′; RP, 5′-GACTTAAGGTGACGCAGGACTC-3′. Reaction conditions were 10 min at 95 °C, followed by 45 cycles of 94 °C for 10 s, 55 °C for 15 s, 72 °C for 15 s. At least three biological replicates were analyzed for each experiment. Products were also analyzed by agarose gel electrophoresis.
Statistics
The data were analyzed by Student's t tests, and significant differences are defined as those with p of 0.05 or less.
Author contributions
Y. K., A. R. G., and S. S. conceived the primary scientific question. Y. K. and A. R. G. designed the experiments. Y. K. performed the mutant genotyping, generated the rescued cell line, and characterized the mutants and their photoheterotrophic growth. Y. K. also produced the GFP-tagged lines and analyzed their localization. W. H. analyzed the conserved cysteine residues of the proteins, characterized the photoautotrophic growth, and analyzed PSI and PSII abundance. S. C., Y. K., and S. S. analyzed the photosynthetic activities. A. R. G., Y. K., S. S., W. H., S. C., A. I., and E. S.-L. analyzed the results. Y. K. wrote the paper with the help of A. R. G., A. I., S. C., S. S., W. H., and E. S.-L.
Supplementary Material
Acknowledgments
We thank Dr. Luke Mackinder and Dr. Silvia Ramundo, from the laboratory of Martin Jonikas, for the pRam118 plasmid, which was used for protein expression.
This work was supported by the Carnegie Institution for Science and by United States Department of Energy Grants 0000229381 and 0000235290. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2 and Figs. S1–S9.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- CBBC
- Calvin–Benson–Bassham cycle
- PET
- photosynthetic electron transport
- ROS
- reactive oxygen species
- AOX
- alternative oxidase
- TCA
- tricarboxylic acid
- PSI and PSII
- photosystem I and II, respectively
- CEF
- cyclic electron flow
- mTERF
- mitochondrial transcription termination factor
- HL
- high light
- LL
- low light
- CIB
- cassette containing internal barcodes 1
- qPCR
- quantitative PCR
- TAP
- Tris acetate phosphate
- TP
- Tris phosphate
- triose-P
- triose phosphate
- MAL
- malate
- OAA
- oxaloacetate
- MDH
- malate dehydrogenase
- gDNA
- genomic DNA
- DCMU
- 3-(3,4-dichlorophenyl)-1,1-dimethylurea
- HA
- hydroxylamine
- Cyt
- cytochrome.
References
- 1. Hohmann-Marriott M. F., and Blankenship R. E. (2011) Evolution of photosynthesis. Annu. Rev. Plant Biol. 62, 515–548 10.1146/annurev-arplant-042110-103811 [DOI] [PubMed] [Google Scholar]
- 2. Niyogi K. K. (2000) Safety valves for photosynthesis. Curr. Opin. Plant Biol. 3, 455–460 10.1016/S1369-5266(00)00113-8 [DOI] [PubMed] [Google Scholar]
- 3. Saroussi S., Sanz-Luque E., Kim R. G., and Grossman A. R. (2017) Nutrient scavenging and energy management: acclimation responses in nitrogen and sulfur deprived Chlamydomonas. Curr. Opin. Plant Biol. 39, 114–122 10.1016/j.pbi.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 4. Erickson E., Wakao S., and Niyogi K. K. (2015) Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J. 82, 449–465 10.1111/tpj.12825 [DOI] [PubMed] [Google Scholar]
- 5. Dang K. V., Plet J., Tolleter D., Jokel M., Cuiné S., Carrier P., Auroy P., Richaud P., Johnson X., Alric J., Allahverdiyeva Y., and Peltier G. (2014) Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 26, 3036–3050 10.1105/tpc.114.126375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639 10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- 7. Peltier G., Tolleter D., Billon E., and Cournac L. (2010) Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth. Res. 106, 19–31 10.1007/s11120-010-9575-3 [DOI] [PubMed] [Google Scholar]
- 8. Kramer D. M., and Evans J. R. (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 155, 70–78 10.1104/pp.110.166652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peltier G., Aro E. M., and Shikanai T. (2016) NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu. Rev. Plant Biol. 67, 55–80 10.1146/annurev-arplant-043014-114752 [DOI] [PubMed] [Google Scholar]
- 10. Saroussi S. I., Wittkopp T. M., and Grossman A. R. (2016) The type II NADPH dehydrogenase facilitates cyclic electron flow, energy-dependent quenching, and chlororespiratory metabolism during acclimation of Chlamydomonas reinhardtii to nitrogen deprivation. Plant Physiol. 170, 1975–1988 10.1104/pp.15.02014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noguchi K., and Yoshida K. (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8, 87–99 10.1016/j.mito.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 12. Bailleul B., Berne N., Murik O., Petroutsos D., Prihoda J., Tanaka A., Villanova V., Bligny R., Flori S., and Falconet D., Krieger-Liszkay A., Santabarbara S., Rappaport F., Joliot P., Tirichine L., et al. (2015) Energetic coupling between plastids and mitochondria drives CO assimilation in diatoms. Nature 524, 366–369 10.1038/nature14599 [DOI] [PubMed] [Google Scholar]
- 13. Bennoun P. (1982) Evidence for a respiratory chain in the chloroplast. Proc. Natl. Acad. Sci. U.S.A. 79, 4352–4356 10.1073/pnas.79.14.4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cardol P., Gloire G., Havaux M., Remacle C., Matagne R., and Franck F. (2003) Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol. 133, 2010–2020 10.1104/pp.103.028076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dutilleul C., Driscoll S., Cornic G., De Paepe R., Foyer C. H., and Noctor G. (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol. 131, 264–275 10.1104/pp.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sweetlove L. J., Lytovchenko A., Morgan M., Nunes-Nesi A., Taylor N. L., Baxter C. J., Eickmeier I., and Fernie A. R. (2006) Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 19587–19592 10.1073/pnas.0607751103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schönfeld C., Wobbe L., Borgstädt R., Kienast A., Nixon P. J., and Kruse O. (2004) The nucleus-encoded protein MOC1 is essential for mitochondrial light acclimation in Chlamydomonas reinhardtii. J. Biol. Chem. 279, 50366–50374 10.1074/jbc.M408477200 [DOI] [PubMed] [Google Scholar]
- 18. Lemaire C., Wollman F. A., and Bennoun P. (1988) Restoration of phototrophic growth in a mutant of Chlamydomonas reinhardtii in which the chloroplast atpB gene of the ATP synthase has a deletion: an example of mitochondria-dependent photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 85, 1344–1348 10.1073/pnas.85.5.1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L. T., Zhang Z. S., Gao H. Y., Meng X. L., Yang C., Liu J. G., and Meng Q. W. (2012) The mitochondrial alternative oxidase pathway protects the photosynthetic apparatus against photodamage in Rumex K-1 leaves. BMC Plant Biol. 12, 40 10.1186/1471-2229-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heineke D., Riens B., Grosse H., Hoferichter P., Peter U., Flügge U. I., and Heldt H. W. (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol. 95, 1131–1137 10.1104/pp.95.4.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson X., and Alric J. (2013) Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: metabolic constraints for carbon partitioning between oil and starch. Eukaryot. Cell 12, 776–793 10.1128/EC.00318-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finnegan P. M., Soole K. L., and Umbach A. L. (2004) Alternative mitochondrial electron transport proteins in higher plants. In Plant Mitochondria: From Genome to Function (Day D. A., Millar H., and Whelan J., eds) pp. 163–230, Springer, New York [Google Scholar]
- 23. Moore A. L., Shiba T., Young L., Harada S., Kita K., and Ito K. (2013) Unraveling the heater: new insights into the structure of the alternative oxidase. Annu. Rev. Plant Biol. 64, 637–663 10.1146/annurev-arplant-042811-105432 [DOI] [PubMed] [Google Scholar]
- 24. Affourtit C., Albury M. S., Crichton P. G., and Moore A. L. (2002) Exploring the molecular nature of alternative oxidase regulation and catalysis. FEBS Lett. 510, 121–126 10.1016/S0014-5793(01)03261-6 [DOI] [PubMed] [Google Scholar]
- 25. Mathy G., Cardol P., Dinant M., Blomme A., Gérin S., Cloes M., Ghysels B., DePauw E., Leprince P., Remacle C., Sluse-Goffart C., Franck F., Matagne R. F., and Sluse F. E. (2010) Proteomic and functional characterization of a Chlamydomonas reinhardtii mutant lacking the mitochondrial alternative oxidase 1. J. Proteome Res. 9, 2825–2838 10.1021/pr900866e [DOI] [PubMed] [Google Scholar]
- 26. Vanlerberghe G. C. (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 14, 6805–6847 10.3390/ijms14046805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J., Rajakulendran N., Amirsadeghi S., and Vanlerberghe G. C. (2011) Impact of mitochondrial alternative oxidase expression on the response of Nicotiana tabacum to cold temperature. Physiol. Plant. 142, 339–351 10.1111/j.1399-3054.2011.01471.x [DOI] [PubMed] [Google Scholar]
- 28. Rachmilevitch S., Xu Y., Gonzalez-Meler M. A., Huang B., and Lambers H. (2007) Cytochrome and alternative pathway activity in roots of thermal and non-thermal Agrostis species in response to high soil temperature. Physiol. Plant. 129, 163–174 10.1111/j.1399-3054.2006.00784.x [DOI] [Google Scholar]
- 29. Murakami Y., and Toriyama K. (2008) Enhanced high temperature tolerance in transgenic rice seedlings with elevated levels of alternative oxidase, OsAOX1a. Plant Biotechnol. 25, 361–364 10.5511/plantbiotechnology.25.361 [DOI] [Google Scholar]
- 30. Hilal M., Zenoff A. M., Ponessa G., Moreno H., and Massa E. M. (1998) Saline stress alters the temporal patterns of xylem differentiation and alternative oxidase expression in developing soybean roots. Plant Physiol. 117, 695–701 10.1104/pp.117.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giraud E., Ho L. H., Clifton R., Carroll A., Estavillo G., Tan Y. F., Howell K. A., Ivanova A., Pogson B. J., Millar A. H., and Whelan J. (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 147, 595–610 10.1104/pp.107.115121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pádua M., Aubert S., Casimiro A., Bligny R., Millar A. H., and Day D. A. (1999) Induction of alternative oxidase by excess copper in sycamore cell suspensions. Plant Physiol. Biochem. 37, 131–137 10.1016/S0981-9428(99)80074-6 [DOI] [Google Scholar]
- 33. Maxwell D. P., Wang Y., and McIntosh L. (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc. Natl. Acad. Sci. U.S.A. 96, 8271–8276 10.1073/pnas.96.14.8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rasmusson A. G., Fernie A. R., and van Dongen J. T. (2009) Alternative oxidase: a defence against metabolic fluctuations? Physiol. Plant. 137, 371–382 10.1111/j.1399-3054.2009.01252.x [DOI] [PubMed] [Google Scholar]
- 35. Vanlerberghe G. C., Vanlerberghe A. E., and McIntosh L. (1997) Molecular genetic evidence of the ability of alternative oxidase to support respiratory carbon metabolism. Plant Physiol. 113, 657–661 10.1104/pp.113.2.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris E. H. (2001) Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 363–406 10.1146/annurev.arplant.52.1.363 [DOI] [PubMed] [Google Scholar]
- 37. Merchant S. S., Prochnik S. E., Vallon O., Harris E. H., Karpowicz S. J., Witman G. B., Terry A., Salamov A., Fritz-Laylin L. K., Maréchal-Drouard L., Marshall W. F., Qu L. H., Nelson D. R., Sanderfoot A. A., Spalding M. H., et al. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 10.1126/science.1143609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peers G., Truong T. B., Ostendorf E., Busch A., Elrad D., Grossman A. R., Hippler M., and Niyogi K. K. (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–521 10.1038/nature08587 [DOI] [PubMed] [Google Scholar]
- 39. Mettler T., Mühlhaus T., Hemme D., Schöttler M. A., Rupprecht J., Idoine A., Veyel D., Pal S. K., Yaneva-Roder L., Winck F. V., Sommer F., Vosloh D., Seiwert B., Erban A., Burgos A., et al. (2014) Systems analysis of the response of photosynthesis, metabolism, and growth to an increase in irradiance in the photosynthetic model organism. Chlamydomonas reinhardtii. Plant Cell 26, 2310–2350 10.1105/tpc.114.124537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blaby I. K., Blaby-Haas C. E., Tourasse N., Hom E. F., Lopez D., Aksoy M., Grossman A., Umen J., Dutcher S., and Porter M., King S., Witman G. B., Stanke M., Harris E. H., Goodstein D., et al. (2014) The Chlamydomonas genome project: a decade on. Trends Plant Sci. 19, 672–680 10.1016/j.tplants.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Massoz S., Larosa V., Horrion B., Matagne R. F., Remacle C., and Cardol P. (2015) Isolation of Chlamydomonas reinhardtii mutants with altered mitochondrial respiration by chlorophyll fluorescence measurement. J. Biotechnol. 215, 27–34 10.1016/j.jbiotec.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 42. Takahashi H., Clowez S., Wollman F. A., Vallon O., and Rappaport F. (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat. Commun. 4, 1954 10.1038/ncomms2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardol P., Alric J., Girard-Bascou J., Franck F., Wollman F. A., and Finazzi G. (2009) Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc. Natl. Acad. Sci. U.S.A. 106, 15979–15984 10.1073/pnas.0908111106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleine T., and Leister D. (2015) Emerging functions of mammalian and plant mTERFs. Biochim. Biophys. Acta 1847, 786–797 10.1016/j.bbabio.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 45. Wobbe L., and Nixon P. J. (2013) The mTERF protein MOC1 terminates mitochondrial DNA transcription in the unicellular green alga Chlamydomonas reinhardtii. Nucleic Acids Res. 41, 6553–6567 10.1093/nar/gkt313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen H. M., Baudet M., Cuiné S., Adriano J. M., Barthe D., Billon E., Bruley C., Beisson F., Peltier G., and Ferro M., and Li-Beisson Y. (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: with focus on proteins involved in lipid metabolism. Proteomics 11, 4266–4273 10.1002/pmic.201100114 [DOI] [PubMed] [Google Scholar]
- 47. Baurain D., Dinant M., Coosemans N., and Matagne R. F. (2003) Regulation of the alternative oxidase Aox1 gene in Chlamydomonas reinhardtii: role of the nitrogen source on the expression of a reporter gene under the control of the Aox1 promoter. Plant Physiol. 131, 1418–1430 10.1104/pp.013409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dinant M., Baurain D., Coosemans N., Joris B., and Matagne R. F. (2001) Characterization of two genes encoding the mitochondrial alternative oxidase in. Chlamydomonas reinhardtii. Curr. Genet. 39, 101–108 10.1007/s002940000183 [DOI] [PubMed] [Google Scholar]
- 49. Siedow J. N., Umbach A. L., and Moore A. L. (1995) The active site of the cyanide-resistant oxidase from plant mitochondria contains a binuclear iron center. FEBS Lett. 362, 10–14 10.1016/0014-5793(95)00196-G [DOI] [PubMed] [Google Scholar]
- 50. Moore A. L., Umbach A. L., and Siedow J. N. (1995) Structure-function relationships of the alternative oxidase of plant mitochondria: a model of the active site. J. Bioenerg. Biomembr. 27, 367–377 10.1007/BF02109999 [DOI] [PubMed] [Google Scholar]
- 51. Andersson M. E., and Nordlund P. (1999) A revised model of the active site of alternative oxidase. FEBS Lett. 449, 17–22 10.1016/S0014-5793(99)00376-2 [DOI] [PubMed] [Google Scholar]
- 52. Molen T. A., Rosso D., Piercy S., and Maxwell D. P. (2006) Characterization of the alternative oxidase of Chlamydomonas reinhardtii in response to oxidative stress and a shift in nitrogen source. Physiol. Plant. 127, 74–86 10.1111/j.1399-3054.2006.00643.x [DOI] [Google Scholar]
- 53. Zalutskaya Z., Ostroukhova M., Filina V., and Ermilova E. (2017) Nitric oxide upregulates expression of alternative oxidase 1 in Chlamydomonas reinhardtii. J. Plant Physiol. 219, 123–127 10.1016/j.jplph.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 54. Zalutskaya Z., Lapina T., and Ermilova E. (2015) The Chlamydomonas reinhardtii alternative oxidase 1 is regulated by heat stress. Plant Physiol. Biochem. 97, 229–234 10.1016/j.plaphy.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 55. Zalutskaya Z., Ostroukhova M., and Ermilova E. (2016) The Chlamydomonas alternative oxidase 1 is regulated by cadmium stress: new insights into control of expression. Environ. Exp. Bot. 130, 133–140 10.1016/j.envexpbot.2016.05.015 [DOI] [Google Scholar]
- 56. Ostroukhova M., Zalutskaya Z., and Ermilova E. (2017) New insights into AOX2 transcriptional regulation in. Chlamydomonas reinhardtii. Eur. J. Protistol. 58, 1–8 10.1016/j.ejop.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 57. Uhmeyer A., Cecchin M., Ballottari M., and Wobbe L. (2017) Impaired mitochondrial transcription termination disrupts the stromal redox poise in Chlamydomonas. Plant Physiol. 174, 1399–1419 10.1104/pp.16.00946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McWilliam H., Li W., Uludag M., Squizzato S., Park Y. M., Buso N., Cowley A. P., and Lopez R. (2013) Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41, W597–W600 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calhoun L. N., and Kwon Y. M. (2011) Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. J. Appl. Microbiol. 110, 375–386 10.1111/j.1365-2672.2010.04890.x [DOI] [PubMed] [Google Scholar]
- 60. Andrews S. C. (2010) The ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys. Acta 1800, 691–705 10.1016/j.bbagen.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 61. Berthold D. A., and Stenmark P. (2003) Membrane-bound diiron carboxylate proteins. Annu. Rev. Plant Biol. 54, 497–517 10.1146/annurev.arplant.54.031902.134915 [DOI] [PubMed] [Google Scholar]
- 62. Yang F., Vought B. W., Satterlee J. S., Walker A. K., Jim Sun Z., Watts J. L., DeBeaumont R., Saito R. M., Hyberts S. G., and Yang S., Macol C., Iyer L., Tjian R., van den Heuvel S., Hart A. C., et al. (2006) An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature 442, 700–704 10.1038/nature04942 [DOI] [PubMed] [Google Scholar]
- 63. Albury M. S., Elliott C., and Moore A. L. (2009) Towards a structural elucidation of the alternative oxidase in plants. Physiol. Plant. 137, 316–327 10.1111/j.1399-3054.2009.01270.x [DOI] [PubMed] [Google Scholar]
- 64. Berthold D. A., Andersson M. E., and Nordlund P. (2000) New insight into the structure and function of the alternative oxidase. Biochim. Biophys. Acta 1460, 241–254 10.1016/S0005-2728(00)00149-3 [DOI] [PubMed] [Google Scholar]
- 65. Selinski J., Hartmann A., Deckers-Hebestreit G., Day D. A., Whelan J., and Scheibe R. (2018) Alternative oxidase isoforms are differentially activated by tricarboxylic acid cycle intermediates. Plant Physiol. 176, 1423–1432 10.1104/pp.17.01331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selinski J., Hartmann A., Höfler S., Deckers-Hebestreit G., and Scheibe R. (2016) Refined method to study the posttranslational regulation of alternative oxidases from Arabidopsis thaliana in vitro. Physiol. Plant. 157, 264–279 10.1111/ppl.12418 [DOI] [PubMed] [Google Scholar]
- 67. Selinski J., Hartmann A., Kordes A., Deckers-Hebestreit G., Whelan J., and Scheibe R. (2017) Analysis of posttranslational activation of alternative oxidase isoforms. Plant Physiol. 174, 2113–2127 10.1104/pp.17.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Djajanegara I., Holtzapffel R., Finnegan P. M., Hoefnagel M. H., Berthold D. A., Wiskich J. T., and Day D. A. (1999) A single amino acid change in the plant alternative oxidase alters the specificity of organic acid activation. FEBS Lett. 454, 220–224 10.1016/S0014-5793(99)00808-X [DOI] [PubMed] [Google Scholar]
- 69. Holtzapffel R. C., Castelli J., Finnegan P. M., Millar A. H., Whelan J., and Day D. A. (2003) A tomato alternative oxidase protein with altered regulatory properties. Biochim. Biophys. Acta 1606, 153–162 10.1016/S0005-2728(03)00112-9 [DOI] [PubMed] [Google Scholar]
- 70. Grant N., Onda Y., Kakizaki Y., Ito K., Watling J., and Robinson S. (2009) Two Cys or not two Cys? That is the question; alternative oxidase in the thermogenic plant sacred Lotus. Plant Physiol. 150, 987–995 10.1104/pp.109.139394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Whelan J., and Glaser E. (1997) Protein import into plant mitochondria. Plant Mol. Biol. 33, 771–789 10.1023/A:1005755505738 [DOI] [PubMed] [Google Scholar]
- 72. Tardif M., Atteia A., Specht M., Cogne G., Rolland N., Brugière S., Hippler M., Ferro M., Bruley C., and Peltier G., Vallon O., and Cournac L. (2012) PredAlgo: a new subcellular localization prediction tool dedicated to green algae. Mol. Biol. Evol. 29, 3625–3639 10.1093/molbev/mss178 [DOI] [PubMed] [Google Scholar]
- 73. Li X., Zhang R., Patena W., Gang S. S., Blum S. R., Ivanova N., Yue R., Robertson J. M., Lefebvre P. A., Fitz-Gibbon S. T., Grossman A. R., and Jonikas M. C. (2016) An indexed, mapped mutant library enables reverse genetics studies of biological processes in. Chlamydomonas reinhardtii. Plant Cell 28, 367–387 10.1105/tpc.15.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baker N. R. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 10.1146/annurev.arplant.59.032607.092759 [DOI] [PubMed] [Google Scholar]
- 75. Apel K., and Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 76. Mittler R., Vanderauwera S., Gollery M., and Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 77. Triantaphylidès C., and Havaux M. (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 14, 219–228 10.1016/j.tplants.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 78. Krieger-Liszkay A., Fufezan C., and Trebst A. (2008) Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 98, 551–564 10.1007/s11120-008-9349-3 [DOI] [PubMed] [Google Scholar]
- 79. Ogilby P. R. (2010) Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 39, 3181–3209 10.1039/b926014p [DOI] [PubMed] [Google Scholar]
- 80. Rhoads D. M., and Subbaiah C. C. (2007) Mitochondrial retrograde regulation in plants. Mitochondrion 7, 177–194 10.1016/j.mito.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 81. Kromer S. (1995) Respiration during photosynthesis. Annu. Rev. Plant Biol. 46, 45–70 10.1146/annurev.pp.46.060195.000401 [DOI] [Google Scholar]
- 82. Vishwakarma A., Bashyam L., Senthilkumaran B., Scheibe R., and Padmasree K. (2014) Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana. Plant Physiol. Biochem. 81, 44–53 10.1016/j.plaphy.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 83. Vishwakarma A., Tetali S. D., Selinski J., Scheibe R., and Padmasree K. (2015) Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in. Arabidopsis thaliana. Ann. Bot. 116, 555–569 10.1093/aob/mcv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salinas T., Larosa V., Cardol P., Maréchal-Drouard L., and Remacle C. (2014) Respiratory-deficient mutants of the unicellular green alga Chlamydomonas: a review. Biochimie 100, 207–218 10.1016/j.biochi.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 85. Yoshida K., Watanabe C. K., Hachiya T., Tholen D., Shibata M., Terashima I., and Noguchi K. (2011) Distinct responses of the mitochondrial respiratory chain to long-and short-term high-light environments in. Arabidopsis thaliana. Plant Cell Environ. 34, 618–628 10.1111/j.1365-3040.2010.02267.x [DOI] [PubMed] [Google Scholar]
- 86. Florez-Sarasa I., Noguchi K., Araújo W. L., Garcia-Nogales A., Fernie A. R., Flexas J., and Ribas-Carbo M. (2016) Impaired cyclic electron flow around photosystem I disturbs high-light respiratory metabolism. Plant Physiol. 172, 2176–2189 10.1104/pp.16.01025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dahal K., Martyn G. D., and Vanlerberghe G. C. (2015) Improved photosynthetic performance during severe drought in Nicotiana tabacum overexpressing a nonenergy conserving respiratory electron sink. New Phytol. 208, 382–395 10.1111/nph.13479 [DOI] [PubMed] [Google Scholar]
- 88. Brettel K., and Leibl W. (2001) Electron transfer in photosystem I. Biochim. Biophys. Acta 1507, 100–114 10.1016/S0005-2728(01)00202-X [DOI] [PubMed] [Google Scholar]
- 89. Dahal K., Wang J., Martyn G. D., Rahimy F., and Vanlerberghe G. C. (2014) Mitochondrial alternative oxidase maintains respiration and preserves photosynthetic capacity during moderate drought in. Nicotiana tabacum. Plant Physiol. 166, 1560–1574 10.1104/pp.114.247866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Selinski J., and Scheibe R. (2018) Malate valves: old shuttles with new perspectives. Plant Biol. (Stuttg.) 10.1111/plb.12869 10.1111/plb.12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang Q. J., Sun H., Dong Q. L., Sun T. Y., Jin Z. X., Hao Y. J., and Yao Y. X. (2016) The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotechnol. J. 14, 1986–1997 10.1111/pbi.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Haferkamp I., Fernie A. R., and Neuhaus H. E. (2011) Adenine nucleotide transport in plants: much more than a mitochondrial issue. Trends Plant Sci. 16, 507–515 10.1016/j.tplants.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 93. Haferkamp I., and Schmitz-Esser S. (2012) The plant mitochondrial carrier family: functional and evolutionary aspects. Front. Plant Sci. 3, 2 10.3389/fpls.2012.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Polukhina I., Fristedt R., Dinc E., Cardol P., and Croce R. (2016) Carbon supply and photoacclimation cross talk in the green alga Chlamydomonas reinhardtii. Plant Physiol. 172, 1494–1505 10.1104/pp.16.01310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Florez-Sarasa I., Flexas J., Rasmusson A. G., Umbach A. L., Siedow J. N., and Ribas-Carbo M. (2011) In vivo cytochrome and alternative pathway respiration in leaves of Arabidopsis thaliana plants with altered alternative oxidase under different light conditions. Plant Cell Environ. 34, 1373–1383 10.1111/j.1365-3040.2011.02337.x [DOI] [PubMed] [Google Scholar]
- 96. Yoshida K., Terashima I., and Noguchi K. (2007) Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiol. 48, 606–614 10.1093/pcp/pcm033 [DOI] [PubMed] [Google Scholar]
- 97. Kropat J., Hong-Hermesdorf A., Casero D., Ent P., Castruita M., Pellegrini M., Merchant S. S., and Malasarn D. (2011) A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 66, 770–780 10.1111/j.1365-313X.2011.04537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang W., Catalanotti C., D'Adamo S., Wittkopp T. M., Ingram-Smith C. J., Mackinder L., Miller T. E., Heuberger A. L., Peers G., Smith K. S., Jonikas M. C., Grossman A. R., and Posewitz M. C. (2014) Alternative acetate production pathways in Chlamydomonas reinhardtii during dark anoxia and the dominant role of chloroplasts in fermentative acetate production. Plant Cell 26, 4499–4518 10.1105/tpc.114.129965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neupert J., Karcher D., and Bock R. (2009) Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 57, 1140–1150 10.1111/j.1365-313X.2008.03746.x [DOI] [PubMed] [Google Scholar]
- 100. Mackinder L. C., Meyer M. T., Mettler-Altmann T., Chen V. K., Mitchell M. C., Caspari O., Freeman Rosenzweig E. S., Pallesen L., Reeves G., Itakura A., Roth R., Sommer F., Geimer S., Mühlhaus T., Schroda M., et al. (2016) A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proc. Natl. Acad. Sci. U.S.A. 113, 5958–5963 10.1073/pnas.1522866113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gibson D. G., Young L., Chuang R. Y., Venter J. C., Hutchison C. A. 3rd, and Smith H. O. (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 102. Rasala B. A., Barrera D. J., Ng J., Plucinak T. M., Rosenberg J. N., Weeks D. P., Oyler G. A., Peterson T. C., Haerizadeh F., and Mayfield S. P. (2013) Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant J. 74, 545–556 10.1111/tpj.12165 [DOI] [PubMed] [Google Scholar]
- 103. Heinnickel M., Kim R. G., Wittkopp T. M., Yang W., Walters K. A., Herbert S. K., and Grossman A. R. (2016) Tetratricopeptide repeat protein protects photosystem I from oxidative disruption during assembly. Proc. Natl. Acad. Sci. U.S.A. 113, 2774–2779 10.1073/pnas.1524040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Porra R., Thompson W., and Kriedemann P. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394 10.1016/S0005-2728(89)80347-0 [DOI] [Google Scholar]
- 105. Dietzel L., Bräutigam K., and Pfannschmidt T. (2008) Photosynthetic acclimation: state transitions and adjustment of photosystem stoichiometry–functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 275, 1080–1088 10.1111/j.1742-4658.2008.06264.x [DOI] [PubMed] [Google Scholar]
- 106. Clowez S., Godaux D., Cardol P., Wollman F. A., and Rappaport F. (2015) The involvement of hydrogen-producing and ATP-dependent NADPH-consuming pathways in setting the redox poise in the chloroplast of Chlamydomonas reinhardtii in anoxia. J. Biol. Chem. 290, 8666–8676 10.1074/jbc.M114.632588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Maxwell K., and Johnson G. N. (2000) Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- 108. Aksoy M., Pootakham W., and Grossman A. R. (2014) Critical function of a Chlamydomonas reinhardtii putative polyphosphate polymerase subunit during nutrient deprivation. Plant Cell 26, 4214–4229 10.1105/tpc.114.129270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012) Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.