Figure 7.
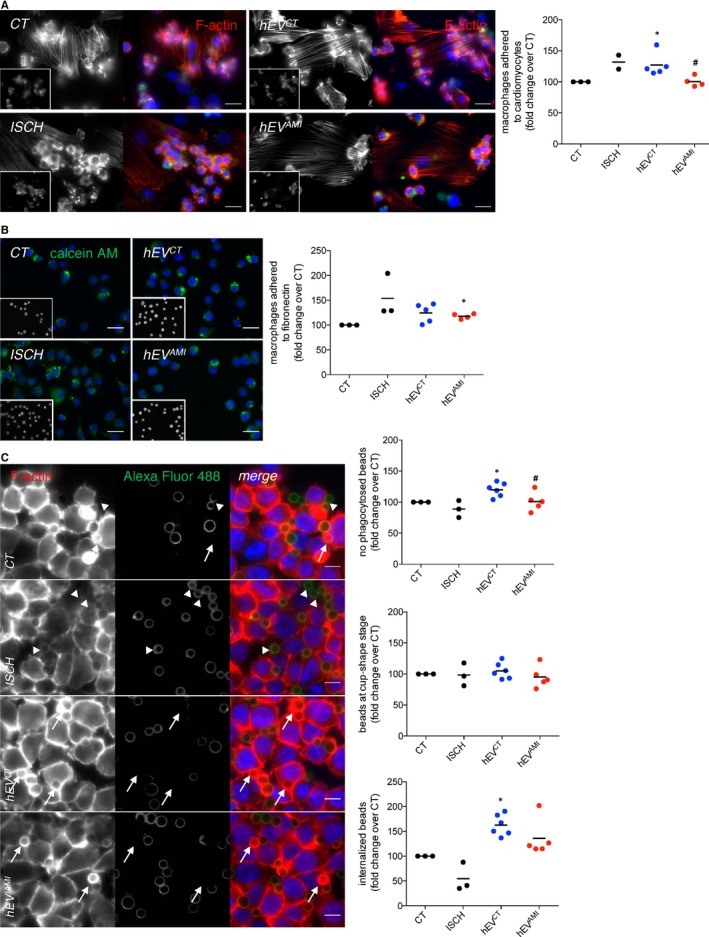
Circulating human EVs modulate cell‐cell, cell‐matrix adhesion and phagocytic activity of macrophages. (A) Macrophages were labelled with 1.5 μmol/L calcein‐AM (insets; green) and further incubated with previously adherent H9c2 cells for 1 hour, with the addition of hEVCT or hEVAMI. Simulated ischaemia was performed for 1 hour. F‐actin was stained with Rhodamine‐Phalloidin (red), and nuclei were stained with DAPI (blue). Scale bars 20 μm. Graph depicts the number of adherent macrophages. *P < 0.05 vs CT, # P < 0.05 vs EVCT (n = 2‐5). (B) Calcein‐AM‐labelled macrophages (green) were added on top of a fibronectin‐based matrix, in the presence of hEVCT or hEVAMI. Simulated ischaemia was performed for 1 hour. Nuclei were stained with DAPI (insets; blue). Scale bars 20 μm. Graph depicts the number of adherent macrophages. *P < 0.05 vs CT (n = 2‐5). (C) Macrophages were incubated with opsonized latex beads for 20 minutes (ratio of 10 beads/cell). Non‐internalized beads were labelled with Alexa Fluor 488 antibodies (green). F‐actin was stained with Rhodamine‐Phalloidin (red). Graphs depict the total number of beads/phagocytic macrophages, represented as percentage of fold change over control (CT), the number of beads within cup‐shaped unsealed nascent phagosomes (positive Alexa Fluor 488 staining, arrow heads) and the number of beads in sealed phagosomes (negative Alexa Fluor 488 staining, arrows). Nuclei were stained with DAPI (blue). Scale bars 10 μm. *P < 0.05 vs CT, # P < 0.05 vs EVCT (n = 3‐6)
