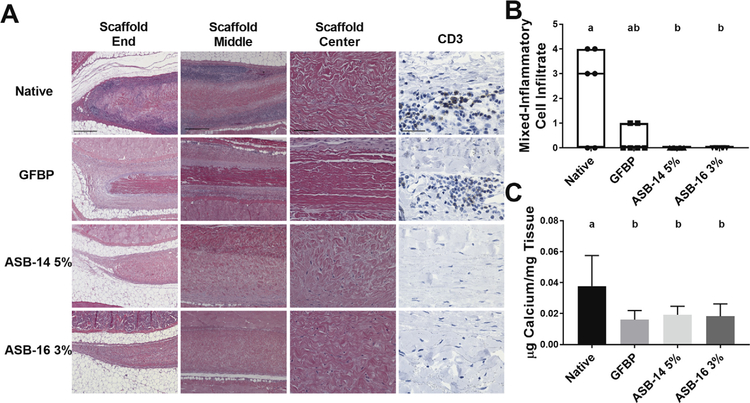
Fig. 4.
Antigen removal significantly reduced recipient graft-specific cell-mediated adaptive immune response. Representative H&E stained histological images of the end (100×), middle (100×), and center (200×) of explanted scaffolds (A). Native bovine pericardium resulted in robust cell-mediated adaptive response (lymphocytes in dark purple regions) at both the end and middle of the scaffold. Lymphocytic infiltration was reduced in glutaraldehyde-fixed bovine pericardium (GFBP) compared to native tissue, although still present at the scaffold ends. Conversely, lymphocytic infiltration was absent in all locations for either ASB-14 5% or ASB-16 3% scaffolds (A). CD3 staining (400×) confirmed that cells observed on H&E staining are lymphocytes and further demonstrated absence of cell-mediated response towards ASB-14 5% or ASB-16 3% scaffolds (A). Blinded review of H&E images by a board certified veterinary pathologist categorized inflammatory cell infiltration on a semi-quantitative scale and confirmed that native tissue stimulated significantly more immune cell response than ASB-14 5% or ASB- 16 3% (B). Calcium deposition was significantly greater in native bovine pericardium explants than in GFBP, ASB-14 5%, and ASB-16 3% (C). Scale bar represents 500 μm (scaffold end and middle images), 100 μm (scaffold center), and 100 μm (CD3). n = 6 per group. Groups not connected by the same lower case letter are statistically significantly different. All data represent the mean ± s.d. except pathology results, which were plotted as median (denoted by the thick line), inter-quartile range (25th–75th percentile), maximum (top whisker) and minimum (bottom whisker).