Abstract
Background:
Autoantibodies against GPIHBP1, the endothelial cell transporter for lipoprotein lipase (LPL), cause severe hypertriglyceridemia (“GPIHBP1 autoantibodies syndrome”). Affected patients have low serum GPIHBP1 and LPL levels. We report the development of a sensitive and specific ELISA, suitable for routine clinical use, to detect GPIHBP1 autoantibodies in serum and plasma.
Methods:
Serum and plasma samples were added to wells of an ELISA plate that had been coated with recombinant human GPIHBP1. GPIHBP1 autoantibodies bound to GPIHBP1 were detected with an HRP-labeled antibody against human immunoglobulin. Sensitivity, specificity, and reproducibility of the ELISA was evaluated with plasma or serum samples from patients with the GPIHBP1 autoantibody syndrome.
Results:
A solid-phase ELISA to detect and quantify GPIHBP1 autoantibodies in human plasma and serum was developed. Spiking recombinant human GPIHBP1 into the samples reduced the ability of the ELISA to detect GPIHBP1 autoantibodies. The ELISA is reproducible and sensitive; it can detect GPIHBP1 autoantibodies in samples diluted by more than 1,000-fold.
Conclusion:
We developed a sensitive and specific ELISA for detecting GPIHBP1 autoantibodies in human serum and plasma; this assay will make it possible to rapidly diagnose the GPIHBP1 autoantibody syndrome.
Keywords: GPIHBP1 autoantibodies, LPL, lipoprotein lipase, lipolysis, autoimmune disease, ELISA
1. Introduction
GPIHBP1 (Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1) is a key player in intravascular lipolysis [1, 2]. GPIHBP1 is expressed exclusively on capillary endothelial cells, and its main function is to bind lipoprotein lipase (LPL) within the subendothelial spaces and shuttle it across endothelial cells to its site of action in the capillary lumen [1,2]. GPIHBP1 loss-of-function mutations abolish the ability of GPIHBP1 to bind and transport LPL, markedly reducing levels of LPL in capillaries and resulting in severe hypertriglyceridemia (chylomicronemia) and a substantial risk for acute pancreatitis [3–8]. Patients with GPIHBP1 mutations invariably have low levels of GPIHBP1 in serum and low levels of LPL in the pre- and post-heparin plasma (consistent with reduced delivery of LPL to the capillary lumen) [3–8].
Recent studies have unequivocally demonstrated that GPIHBP1 autoantibodies are responsible for some acquired cases of chylomicronemia [9, 10]. Beigneux and coworkers identified six patients with unexplained chylomicronemia with GPIHBP1 autoantibodies (“GPIHBP1 autoantibodies syndrome”) [9, 10]. They demonstrated that the GPIHBP1 autoantibodies blocked the ability of GPIHBP1 to bind and transport LPL. Like patients with inherited forms of GPIHBP1 deficiency, patients with the GPIHBP1 autoantibodies syndrome have low levels of LPL in their plasma [9, 10]. The plasma levels of GPIHBP1 are also low because the GPIHBP1 autoantibodies interfere with the detection of GPIHBP1 in the plasma [9]. Many but not all patients with the GPIHBP1 autoantibody syndrome have clinical or serological evidence for autoimmune disease [9].
At the current time, assays for GPIHBP1 autoantibodies are not routinely available. Here, we describe the development of a highly sensitive and specific ELISA for the detection of GPIHBP1 autoantibodies, making it possible for clinicians to diagnose the GPIHBP1 autoantibody syndrome in an unequivocal fashion.
2. Materials and Methods
2.1. Blood samples
Blood samples were obtained according to the principles outlined in the Declaration of Helsinki. Sera from 28 healthy Japanese volunteers in the non-fasting state (aged 23-60, male 19, female 9) were obtained at Immuno-Biological Laboratory (Fujioka, Japan). We also examined de-identified archived plasma samples from patients with loss-of-function mutations in GPIHBP1. [8, 9, 11] Those samples had been sent to UCLA without identifiers [9] and were deemed exempt from institutional review board approval. As positive controls, we used archived serum and plasma samples from documented cases of GPIHBP1 autoantibody syndrome about the details already reported [9, 10]. Briefly, the patients with GPIHBP1 autoantibodies who had chylomicronemia had received a diagnosis of an autoimmune disease (SLE or Sjögren’s syndrome). In such patients, autoantibodies against many proteins can develop, and our data indicate that GPIHBP1 is one of those proteins. Because of the transfer of maternal autoantibodies, an infant who was bom to a mother with SLE was found to have neonatal lupus (characterized by cutaneous lesions and cardiac conduction abnormalities). On the basis of our data, chylomicronemia is a potential finding in infants born to mothers with SLE. Of the other patients with GPIHBP1 autoantibodies, two had no evidence of rheumatologic disease.
2.2. An ELISA system to detect GPIHBP1 autoantibodies
A cDNA encoding a soluble version of FLAG-tagged human GPIHBP1 (amino acids 21–150) was synthesized by FASMAC (Tokyo), expressed in CHO cells, and human GPIHBP1 protein was then purified from the medium with a FLAG M2 column. Plasma or serum samples (diluted 1000-fold) were added to wells of a 96-well ELISA plate that had been coated with the purified GPIHBP1 (50 ng/well, 1 h at room temperature) (Fig. 1). After washing, 50 ng of HRP-labeled goat anti-human IgG was added to the wells and incubated for 30 min at room temperature. After washing, 100 μl of TMB substrate was added to the wells. After 15 min, the reaction was stopped by adding 100 μl of 2 M sulfuric acid to the wells. The absorbance (OD) was read at 450 nm (Fig. 1).
Fig. 1.
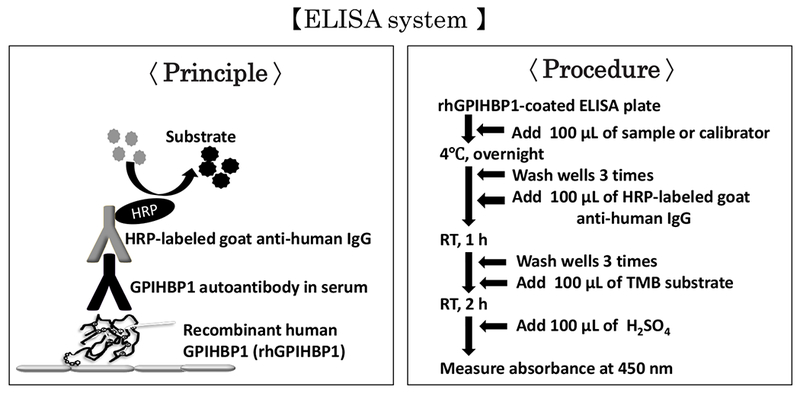
An immunoassay for quantifying autoantibodies against GPIHBP1. Human plasma or serum is added to wells of an ELISA plate that had been coated with recombinant human GPIHBP1. After washing, human immunoglobulins (IgGs) bound to the recombinant human GPIHBP1 are detected with an HRP-labeled goat anti-human IgG antibody.
Using a plasma sample from a well-documented case of the GPIHBP1 autoantibodies syndrome [9, 10], we set a GPIHBP1 autoantibodies titer at 1 unit (U)/ml when a 1:1000 dilution of a sample by PBS yielded an OD of 2.0 (at 450 nm) (Fig. 2A). Further 1:2 serial dilutions of the sample by PBS revealed that the ELISA could detect GPIHBP1 autoantibodies when the titer fell within the range of 0.03 U/ml to 1 U/ml (Fig. 2A), and that the assay was linear up to a 1:4000 dilution of the plasma samples (Fig. 2B).
Fig. 2.
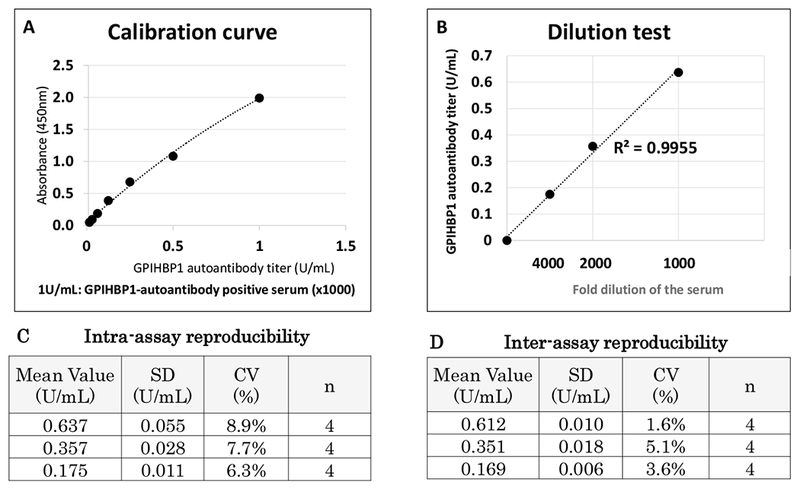
The GPIHBP1 autoantibodies ELISA. A GPIHBP1 autoantibodies–positive serum was used for the calibration curve, dilution linearity, and intra-, and inter-assay accuracy. (A) Calibration curve with a GPIHBP1 autoantibodies–positive serum (1 U/ml). (B) Dilution test, 1000- to 4000-fold dilution. (C) Intra-assay reproducibility. (D) Inter-assay reproducibility.
2.2. Measurements of triglycerides and GPIHBP1 in serum and plasma
Plasma triglyceride levels were determined with enzymatic assay (Quick Neo TG II, Shino-Test Corporation, Tokyo). Plasma GPIHBP1 levels were measured with a solid-phase monoclonal antibody–based sandwich ELISA (Immuno-Biological Laboratories, Fujioka, Japan) [12]. For the GPIHBP1 ELISA, serum and plasma were diluted 10-fold in PBS; 100 μL of the diluted samples were added to wells of the 96-well ELISA plate.
3. Results
3.1. Plasma sample processing
The serum or plasma samples were separated by centrifugation (2500 ×g) at 4 °C for 10 min and kept frozen at −80 °C until analysis. Heparin administration (30 units/kg) was performed to confirm the low LPL concentration in 3 GPIHBP1 autoantibodies positive cases.
3.2. Performance of the GPIHBP1 autoantibodies ELISA
Detection of GPIHBP1 autoantibodies titers was linear up to 1 U/ml (Fig. 2 A) and the dilution to over 4000 fold (Fig. 2B). The analytical detection limit was estimated as the equal to the mean absorbance of 10 replicates of the zero calibrator plus 3 SD. The detection limit was as low as 0.03 U/ml. The intra- and inter-assay variation of the GPIHBP1 autoantibodies ELISA was measured with three quality control (QC) samples, corresponding to the high, middle, and low region of the calibration curve. The intra-assay variation was calculated from four repeated measurements of the three QC samples in a single plate. The inter-assay variation was calculated by measuring each QC sample across four different plates. The intra-assay variation was 8.9% for the high, 7.7% for the middle, and 6.3% for the low QC sample (Fig. 2C). The inter-assay variation was 1.6% for the high, 5.1% for the middle, and 3.6% for the low QC sample (Fig. 2D). The performance of our GPIHBP1 autoantibodies ELISA was very similar whether serum or plasma was used for the QC samples.
To test the specificity of our ELISA for GPIHBP1 autoantibodies, we spiked plasma samples from six cases of GPIHBP1 autoantibody syndrome [9] with known amounts of human GPIHBP1 (final human GPIHBP1 concentrations of 21, 42, 85, 170, or 340 ng/ml) before adding the samples to the wells of a GPIHBP1 autoantibody ELISA plate (Fig. 3). Adding increasing amounts of recombinant GPIHBP1 to the plasma samples decreased, in a dose-dependent manner, the ability to detect GPIHBP1 autoantibodies in the sample, indicating that the spiked human GPIHBP1 competed with the immobilized GPIHBP1 for autoantibodies binding (Fig. 3).
Fig. 3.
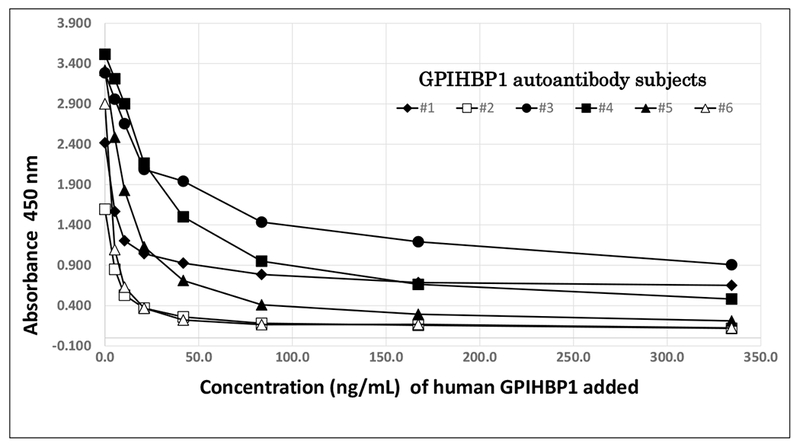
Inhibition of the autoantibodies immunoassay by recombinant human GPIHBP1. Serum samples from six cases of the GPIHBP1 autoantibodies syndrome were spiked with increasing amounts of recombinant human GPIHBP1 (21, 42, 85, 170, or 340 ng/ml) before being added to wells of a GPIHBP1 autoantibodies ELISA plate. Plotted are the absorbance at 450 nm measured in all spiked samples.
No effect of heparin administration was found on the detection of GPIHBP1 autoantibodies, as we often need to determine the LPL concentration in post-heparin plasma in cases with GPIHBP1 autoantibodies positive.
3.3. Establishing a cut-off value for GPIHBP1 autoantibodies
To establish a cut-off value for GPIHBP1 autoantibodies positivity, we compared serum levels of GPIHBP1 in 28 healthy control subjects and 21 hypertriglyceridemic subjects, including LPL-delicient patients and patients functionally deficient in GPIHBP1 (due to either loss-of-function mutations in GPIHBP1 or GPIHBP1 autoantibodies) [9]. Healthy control subjects had plasma triglyceride levels below 150 mg/dl and serum GPIHBP1 levels ranging from 600–1600 pg/ml (Fig. 4). The mean GPIHBP1 level was 849 ± 225 pg/ml (a GPIHBP1 level of 260 pg/ml, corresponding to 2.6 standard deviations below the mean). The 21 hypertriglyceridemic subjects had plasma triglyceride levels above 1000 mg/dl; eight of the hypertriglyceridemic subjects had serum GPIHBP1 levels within normal range (>260 pg/ml), while the other 13 hypertriglyceridemic subjects had serum GPIHBP1 levels below 260 pg/ml. All 7 subjects with the GPIHBP1 autoantibody syndrome [9] fell into the latter group.
Fig. 4.
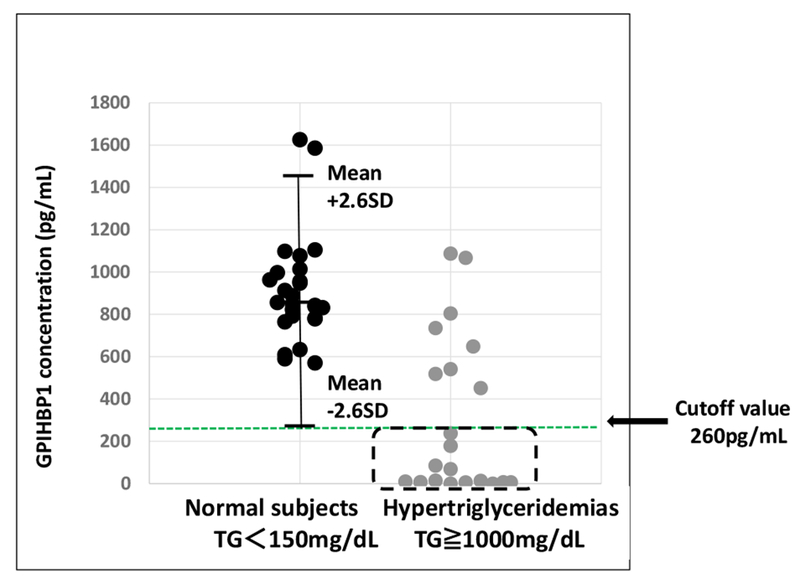
Description of the 13 hypertriglyceridemic samples used to validate the GPIHBP1 autoantibodies ELISA. Twenty-eight samples from normolipemic controls (serum triglyceride levels <150 mg/dl) and 21 samples from hypertriglyceridemic patients (serum triglyceride levels ≥1000 mg/dl) were analyzed for levels of GPIHBP1. In normolipemic samples, the serum levels of GPIHBP1 ranged from 600–1600 pg/ml, with a mean of 849 pg/ml ± 225 (a level of 260 pg/ml corresponds to 2.6 standard deviations below the mean). Eight of the hypertriglyceridemic samples had normal serum GPIHBP1 levels (>260 pg/ml); the remaining 13 samples had abnormally low serum GPIHBP1 levels (<260 pg/ml). The latter group included seven samples from patients with the GPIHBP1 autoantibodies syndrome and six samples from patients who were homozygous for loss-of-function mutations in GPIHBP1 [9].
We then compared GPIHBP1 autoantibodies titers in the 28 samples from healthy control subjects and the 13 subjects with GPIHBP1 levels <260 pg/ml. The GPIHBP1 autoantibodies titer was 23 ± 14 U per ml of plasma (range 9–57 U/ml) in the normal control subjects (Fig. 5). Six of the 13 hypertriglyceridemic subjects also had a GPIHBP1 autoantibodies titer <58 U/ml; however, the GPIHBP1 autoantibodies titers in the seven subjects with the GPIHBP1 autoantibody syndrome were far higher, ranging from 533 to 29,224 U per ml of plasma (Fig. 5). We therefore established 58 U/ml as the cut-off value for “positive” GPIHBP1 autoantibodies.
Fig. 5.
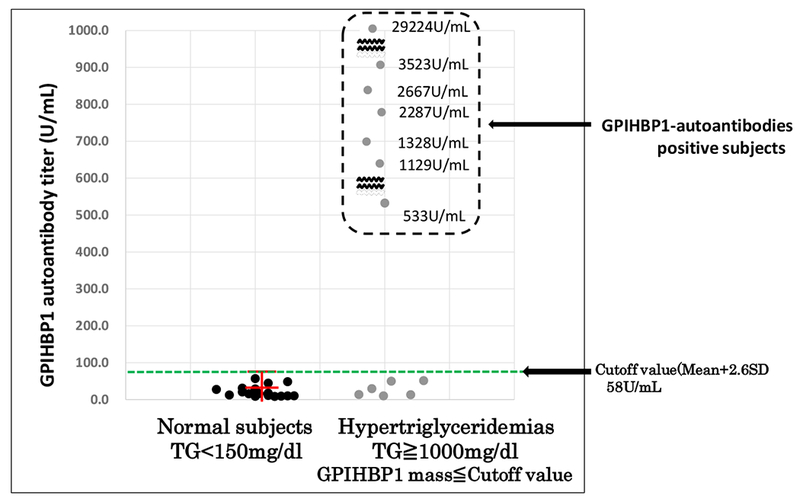
GPIHBP1 autoantibodies titers in 28 normolipemic and 13 hypertriglyceridemic samples. The samples were tested at a 1:1000 dilution. All 28 normolipemic control samples exhibited an OD 450 nm < 0.2, corresponding to a GPIHBP1 autoantibodies titer below 58 U/ml. The six samples from patients with a genetic defect in GPIHBP1 also had a GPIHBP1 autoantibodies titer below 58 U/ml. All seven samples from patients with the GPIHBP1 autoantibodies syndrome had a titer at least 9-times greater than the 58 U/ml cut-off value (ranging from 533–29,224 U/ml).
4. Discussion
We report the development of a solid-phase ELISA for quantifying GPIHBP1 autoantibodies in human serum or plasma. The GPIHBP1 autoantibodies ELISA yields reproducible data and is both specific and sensitive. It is capable of detecting GPIHBP1 autoantibodies titers as low as 0.03 U/ml and is linear up to 1 U/ml. To eliminate the risk of a false positive, it is important to dilute the plasma samples by 1000-fold; this is particularly important for samples with high concentrations of immunoglobulins or samples from patients with autoimmune disease. Samples from patients with the GPIHBP1 autoantibody syndrome had a GPIHBP1 autoantibodies titer at least nine times higher than the 58 U/ml cut-off value in our ELISA. Our ELISA works well on plasma and serum samples, pre- and post-heparin plasma, and both fasted and postprandial samples.
In our studies, spiking recombinant GPIHBP1 into plasma from patients with the GPIHBP1 autoantibody syndrome reduced the ability of our ELISA to detect GPIHBP1 autoantibodies (i.e., there was clearly competition between the spiked GPIHBP1 and the immobilized GPIHBP1 on ELISA wells for autoantibodies binding). This finding indicates that the plasma of patients with the GPIHBP1 autoantibody syndrome contain significant amounts of free autoantibodies (GPIHBP1 autoantibodies not complexed to GPIHBP1). It is unlikely that our ELISA would be capable of detecting GPIHBP1–GPIHBP1 autoantibodies immune complexes in the plasma. In the future, it would be desirable to develop an ELISA for measuring levels of GPIHBP1–GPIHBP1 autoantibodies immune complexes.
LPL autoantibodies have long been proposed as a possible cause of hypertriglyceridemia [13, 14], while the discovery of GPIHBP1 autoantibodies as a cause of hypertriglyceridemia was recent [9, 10]. While we had no difficulty in documenting multiple cases of GPIHBP1 autoantibodies with our ELISA [9, 12], we have yet to identify a single case of LPL autoantibodies (even in samples that were thought to have LPL autoantibodies). In the past, the antigens and antibodies used to detect LPL autoantibodies have not been optimal and have resulted in misleading results [15, 16]. We would be extremely interested in identifying and investigating a bona fide case of chylomicronemia due to LPL autoantibodies.
In conclusion, we have developed a sensitive and specific ELISA for the detection of GPIHBP1 autoantibodies in human serum and plasma. This assay will be quite useful for making a diagnosis of the GPIHBP1 autoantibody syndrome.
Highlights.
GPIHBP1 autoantibodies cause severe hypertriglyceridemia (chylomicronemia).
An ELISA to measure GPIHBP1 autoantibodies in serum or plasma was developed.
The ELISA is sensitive, capable of detecting GPIHBP1 autoantibodies.
This assay is useful for diagnosing the GPIHBP1 autoantibody syndrome.
5. Acknowledgments
This work was supported in part by a Grant-in-Aid (26460640) for General Scientific Research from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan. Also, this work was supported by grants from the NHLBI (HL090553, HL087228, HL139725-01) and a Transatlantic Network Grant from the Leducq Foundation (12CVD04).
Abbreviations:
- LPL
lipoprotein lipase
- GPIHBP1
glycosylphosphatidylinositol-anchored high density lipoprotein binding protein-1
Footnotes
Disclosures
The authors have no disclosures.
7. References
- [1].Beigneux AP, Davies B, Gin P, Weinstein MM, Farber E, Qiao X, Peale P, Bunting S, Walzem RL, Wong JS, Blaner WS, Ding ZM, Melford K, Wongsiriroj N, Shu X, de Sauvage F, Ryan RO, Fong LG, Bensadoun A, Young SG, Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons, Cell Metab. 5 (2007) 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Davies BS, Beigneux AP, Bames RH 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG, GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries, Cell Metab. 12(1) (2010) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beigneux AP, Franssen R, Bensadoun A, Gin P, Melford K, Peter J, Walzem RL, Weinstein MM, Davies BS, Kuivenhoven JA, Kastelein JJ, Fong LG, Dallinga-Thie GM, Young SG, Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase, Arterioscler. Thromb. Vase. Biol 29 (2009) 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Charriere S, Peretti N, Bernard S, Di Filippo M, Sassolas A, Merlin M, Delay M, Debard C, Lefai E, Lachaux A, Moulin P, Marcais C. GPIHBP1 C89F neomutation and hydrophobic C-Terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia, J. Clin. Endocrinol. Metab 96(10) (2011) E1675–E1679. [DOI] [PubMed] [Google Scholar]
- [5].Coca-Prieto I, Kroupa O, Gonzalez-Santos P, Magne J, Olivecrona G, Ehrenborg E, Valdivielso P, Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation, J. Intern. Med 270(3) (2011) 224–228. [DOI] [PubMed] [Google Scholar]
- [6].Franssen R, Young SG, Peelman F, Hertecant J, Sierts JA, Schimmel AWM, Bensadoun A, Kastelein JJP, Fong LG, Dallinga-Thie GM, Beigneux AP, Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects, Circ. Cardiovasc. Genet 3(2) (2010) 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Olivecrona G, Ehrenborg E, Semb H, Makoveichuk E, Lindberg A, Hayden MR, Gin P, Davies BSJ, Weinstein MM, Fong LG, Beigneux AP, Young SG, Olivecrona T, Hernell O, Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia, J. Lipid Res 51(6) (2010) 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Plengpanich W, Young SG, Khovidhunkit W, Bensadoun A, Karnman H, Ploug M, Gardsvoll H, Leung CS, Adeyo O, Larsson M, Muanpetch S, Charoen S, Fong LG, Niramitmahapanya S, Beigneux AP, Multimerization of GPIHBP1 and familial chylomicronemia from a serine-to-cysteine substitution in GPIHBP1’s Ly6 domain, J. Biol. Chem 289(28) (2014) 19491–19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beigneux AP, Miyashita K, Ploug M, Blom DJ, Ai M, Linton MF, Khovidhunkit W, Dufour R, Garg A, McMahon MA, Pullinger CR, Sandoval NP, Hu X, Allan CM, Larsson M, Machida T, Murakami M, Reue K, Tontonoz P, Goldberg IJ, Moulin P, Charriere S, Fong LG, Nakajima K, Young SG, Autoantibodies against GPIHBP1 as a Cause of Hypertriglyceridemia, N. Engl. J. Med 376(17) (2017) 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu X, Dallinga-Thie GM, Hovingh GK, Chang SY, Sandoval NP, Dang TLP, Fukamachi I, Miyashita K, Nakajima K, Murakami M, Fong LG, Ploug M, Young SG, Beigneux AP, GPIHBP1 autoantibodies in a patient with unexplained chylomicronemia, J. Clin. Lipidol 11(4) (2017) 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rios JJ, Shastry S, Jasso J, Hauser N, Garg A, Bensadoun A, Cohen JC, Hobbs HH, Deletion of GPIHBP1 causing severe chylomicronemia, J. Inherit. Metab. Dis 35(3) (2012) 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miyashita K, Fukamachi I, Nagao M, Ishida T, Kobayashi J, Machida T, Nakajima K, Murakami M, Ploug M, Beigneux AP, Young SG, Nakajima K, An enzyme-linked immunosorbent assay for measuring GPIHBP1 levels in human plasma or serum, J. Clin. Lipidol 12(1) (2018) 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kihara S, Matsuzawa Y, Kubo M, Nozaki S, Funahashi T, Yamashita S, Sho N, Tarui S, Autoimmune hyperchylomicronemia, N. Engl. J. Med 320 (1989) 1255–1259. [DOI] [PubMed] [Google Scholar]
- [14].Yoshimura T, Ito M, Sakoda Y, Kobori S, Okamura H, Rare case of autoimmune hyperchylomicronemia during pregnancy, Eur. J. Obstet. Gynecol. Reprod. Biol 76(1) (1998) 49–51. [DOI] [PubMed] [Google Scholar]
- [15].Blom DJ, Marais AD, Severe hypertriglyceridemia in a patient with lupus, Am. J. Med 118(4) (2005) 443–444. [DOI] [PubMed] [Google Scholar]
- [16].Ashraf AP, Beukelman T, Pruneta-Deloche V, Kelly DR, Garg A, Type 1 hyperlipoproteinemia and recurrent acute pancreatitis due to lipoprotein lipase antibody in a young girl with Sjogren’s syndrome, J. Clin. Endocrinol. Metab 96(11) (2011) 3302–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]


