Figure 7.
Proposed Transport Mechanism of the Mitochondrial ADP/ATP Carrier
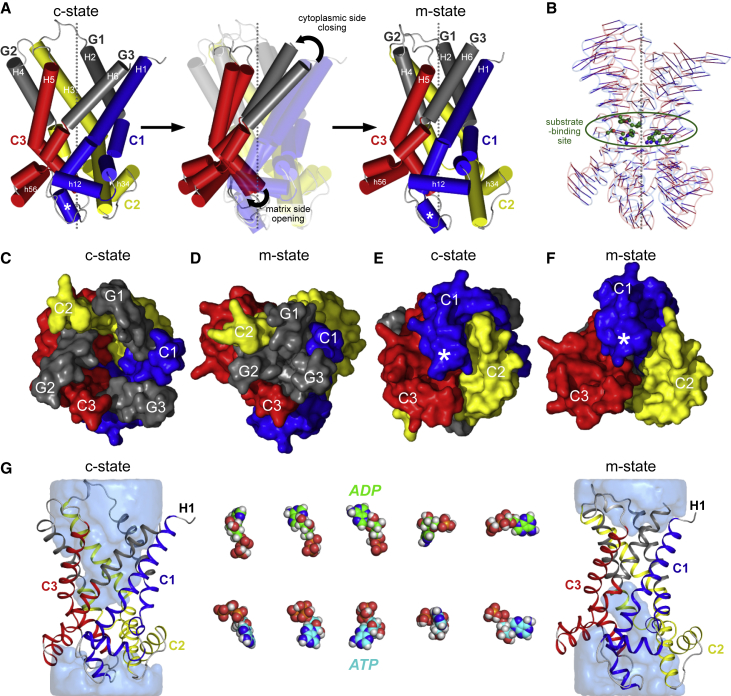
(A) Conformational changes between models of the uninhibited c- and m-states viewed laterally from the membrane, with helices shown as cylinders. The three-fold pseudosymmetry axis is shown as a dotted gray line. Conformational changes between c- and m-states can be described as a rotation of the core elements, coupled with an inward movement of the gate elements. Core elements 1, 2, and 3 are colored by domain in blue, yellow, and red, respectively, and the gate elements are colored gray. The asterisk marks the extra turn of α-helix on the matrix end of H1, seen in fungal ADP/ATP carriers.
(B) Conformational changes between c- and m-states use the substrate-binding site as a fulcrum. Alignment of models of the uninhibited c- (blue) and m-states (red), with lines connecting equivalent Cα positions, generated by RAPIDO (Mosca and Schneider, 2008). Substrate-binding site residues are shown in green ball-and-sticks. The 3-fold pseudosymmetry axis is shown as a dotted gray line.
(C–F) Models of the uninhibited c-state and m-states viewed from the cytoplasmic side of the inner mitochondrial membrane, (C) and (D) respectively, and from the mitochondrial matrix, (E) and (F) respectively, colored and labeled as in (A). Loop regions between H2-H3 and H4-H5 have been omitted to provide a clearer view of the core and gate elements.
(G) Accessibility of the substrate-binding site in the uninhibited carrier models. The carrier is shown as a cartoon colored as in (A). The blue semi-transparent surface shows the internal cavities. ATP and ADP are shown in sphere representation in different views.
See also Video S1.