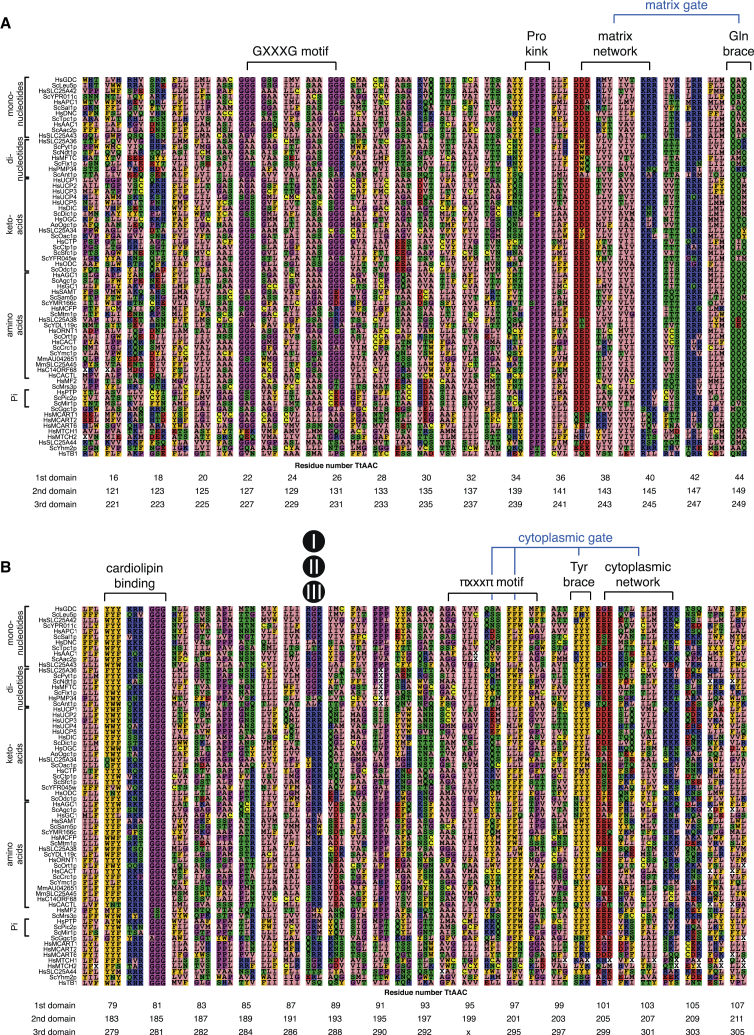
Figure S4.
Alignment of Symmetry-Related Triplets from Yeast and Human Carrier Sequences, Related to Figures 1, 2, 3, 5, and 6
(A) The odd-numbered helices and (B) even-numbered helices of different mitochondrial carriers of Saccharomyces cerevisiae (Sc), Homo sapiens (Hs), Musculus musculus (Mm), and Aspergillus oryzae (Ao). The residues are shown as a triplet of symmetry-related residues of domain 1, 2 and 3 together to emphasize the symmetry in the three-fold repeats of mitochondrial carriers (Robinson et al., 2008). Below the triplets are the corresponding numbers of the residues in TtAac that form the symmetry-related triplet. Amino acids are colored according to their properties as in Figure S1. Indicated above are the functional elements of mitochondrial carriers and the contact points of the substrate binding site in black circles with roman numerals (Robinson and Kunji, 2006). Figure adapted from Figures S1 and S3 from Robinson et al. (2008). Copyright (2008) National Academy of Sciences.