Abstract
Exposure to mine tailings dust from active and abandoned mining operations may be a very significant health hazard, especially to sensitive populations living in arid and semi-arid climates like the desert southwest of the US. It is anticipated that early life exposures during sensitive times of development can lead to adult disease. However, very few studies have investigated the effects of inhalation exposure to real world dusts during lung development. Using a mouse model, we have examined the effect(s) of inhalation of real world mine tailing dusts under three separate conditions: (1) Exposure only during in utero development (exposure of the pregnant moms) (2) exposure only after birth and (3) exposures that occurred continuously during in utero development, through gestation and birth until the mice reached adulthood (28 days old). We found that the most significant changes in lung structure and function were observed in male mice when exposure occurred continuously throughout development. These changes included increased airway hyper-reactivity, increased expression of epithelial to mesenchymal (EMT) transition protein markers and increased expression of cytokines related to eosinophils. The data also indicate that in utero exposures through maternal inhalation can prime the lung of male mice for more severe responses to subsequent postnatal exposures. This may be due to epigenetic alterations in gene regulation, immune response, molecular signaling, and growth factors involved in lung development that may make the neonatal lung more susceptible to continued dust exposure.
Keywords: Mine Tailings Dust, Lung Development, Lung Disease
Introduction
Growth and development requires the temporal and spatial coordinated expression of genes and gene products. During this critical time, in utero and early postnatal exposure to toxicants has the potential to alter organ structure and physiological function, which can be manifested as adult disease (Merkus, 2003; Shi et al, 2007; Wei et al, 2007; Whitsett et al, 2011; Farzan et al 2013; ; Tang et al, 2017). While the potential adverse health outcomes that result from exposures during these development times are recognized, only limited attention has been paid to the effect(s) of environmentally relevant exposures to toxicants during these critical periods of development (Majumdar and Mazumder, 2012; Vahter, 2008) and even fewer studies have examined whether early life inhalation exposures may lead to adult disease. (Chan et al, 2011; Fedulov et al, 2008; Clark et al, 2010; Hsu et al, 2015; Spann et al, 2016). The low humidity and strong winds found in the arid US Southwest make respirable dust levels potentially problematic. The incidence of high dust storms in the Southwestern US has been increasing in recent years and this is especially a serious concern in arid and semi-arid regions due to the increased susceptibility to windborne transport of metal-laden dust particles. This issue can be particularly acute just downwind from mining sites where dusts can contain high levels of metal(oid)s (Csavina et al, 2011). Global climate models predict warmer and drier conditions in the Southwest in the near future that will heighten windblown dust and the abundance of dust-borne particulates (Breshears et al, 2012). Thus, there are immediate and growing needs to evaluate the toxicity of dusts and their components following early life inhalation exposures.
Very few previous reports have evaluated sensitive developmental exposure times following toxicant inhalation. Mauad et al, (2008) evaluated the effects of inhalation of vehicle emissions exposures (urban PM2.5). Animals were housed near a busy roadway and were exposed to emissions prenatally, postnatally, or both pre- and postnatally. Significant alterations in lung surface to volume ratios and lung function were only observed in animals exposed both pre- and postnatally. In a separate study, Thevenot et al, (2013) showed that early postnatal inhalation exposure to radical-containing ultrafine particulate matter resulted in increased expression of epithelial to mesenchymal transition (EMT) markers in 7-day old animals. Alterations in EMT were attributed to the production of reactive oxygen species (ROS). However, the effects of in utero exposures were not examined. The need for continuous developmental exposures to produce adverse outcomes was also observed in rats exposed to sidestream cigarette smoke (Joad et al, 1995). Exposure to sidestream smoke both pre- and postnatally (but not only pre- or only postnatally) resulted in lungs with less compliance, increased airway hyper-reactivity, and had a greater number of pulmonary neuroendocrine cells. A separate report showed increased airway hyper-reactivity with prenatal and early postnatal exposures without increases in pulmonary neuroendocrine cells or mast cells (Joad et al, 1999). Finally, intraperitoneal injection of PM2.5 extracts into pregnant mice resulted in alterations in lung function and induction of EMT markers in 28-day old offspring (Tang et al, 2017). With the exception for a few studies on occupational exposures, little data exist concerning the risk in adults from exposure to metal(oid) containing dusts through inhalation, and the effect of inhalation of metal/metalloid-containing dusts during in utero and early life exposure is not known.
Using metal-containing real world dusts collected from an abandoned mine tailing, we have exposed male and female mice during in utero development alone, postnatally alone or continuous in utero and postnatal exposures. We have found that significant increases in airway reactivity are only seen in male mice and that the greatest changes in lung function and structure occur following continuous exposures. Alterations in male mice include increased pulmonary resistance, increased airway hyperreactivity, increased expression of EMT markers and indications that these changes may be associated with increased reactive oxygen products. The finding that both in utero and early postnatal exposures are required to produce significant alterations in lung structure and function suggests that there are in utero effects caused by the mothers’ inhalation of the toxicant that “primes” the lung of the offspring for subsequent responses to inhaled toxicants after birth. The effects of the in utero exposures only become apparent following a subsequent exposure in the pups after birth.
Methods and Materials
Laboratory Dust Generation and Fractionation
The present inhalation toxicology study focuses on the Iron King and Humboldt Smelter Superfund (IKMSS) site in Dewey-Humboldt, Arizona. In order to collect enough real world respirable dusts for inhalation exposures, a portable dust generator and size fractionation system was developed by engineers working under the University of Arizona Superfund Research Program. Dust was generated from topsoil collected from mine tailings. The dust collection system, dust metal content and size fraction characteristics are given in detail in Gonzales et al (2014). The fine dust produced was effectively comprised of particles smaller than 10 μm, with the majority of particles by number in the range of 0.5–5 μm. Fine dust generated from all samples had a size distribution with a count median diameter around 1.2 μm and a geometric standard deviation of approximately 3 μm as determined by spectrometric size distribution analysis. Dusts collected at the mine tailings have been found to contain significant arsenic (As) and lead (Pb) levels with the highest levels seen in the PM10 size fraction. (Gonzales et al, 2014). Levels of arsenic and lead comprised 1.1% and 1.3% of the total mass, respectively. As and Pb concentrations are the highest in the tailings at the IKMSS site and consistently decreased in the soil samples when comparing all size fractions at increasing distances from the tailings (Stovern et al, 2016).
Animal Inhalation Exposures
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Arizona (Protocol Number 07–140). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Beginning one week before mating, female C57BL/6 mice were exposed to daily dust IKMSS aerosol (PM10) by the Vilnius dust generator (CH Technologies, Westwood, NJ) attached to 9 individual inhalation cages with an in-line real-time aerosol particle measurement device (Casella Microdust Pro Sampler, Casella Measurement, Bedford, UK) with continuous negative feedback dust generation at the flow rate of 2 L/min at a concentration of 4.8 mg/m3 for 30 min/day. This exposure period of 30 min/day was chosen to reduce stress to the mice to achieve a 24-hour time weighted average exposure of 100 μg/m3. The level of dust concentration inside the cages was also measured by a separate device, Dust Track II, which was placed inside the exposure system. The concentration of the dust exposure was adjusted by constant evaluation of the two dust measurement systems.
Four groups of mice were utilized in the study; a control group exposed to ambient air (Control), a group exposed to dust only in utero (gestational development, GD), a group exposed to dust only post-natally (PN), and a combined in utero and post-natally exposed group (GDPN). Pregnant mice were placed in groups and exposed to the dust until they reached gestational time and then were divided into two groups; in utero only group that had their dust exposure stopped when the pups were born and a combined in utero and post-natal exposure group that kept undergoing the dust inhalation exposure. The post-natal group was housed in the animal facility until they reached gestational day 18 and then were transferred to the inhalation cages whereby the dust exposures commenced on the day of birth of the mouse pups. The mouse pups were then exposed on a daily basis. When animals in all groups reached 28 days of age both male and female offspring mice underwent pulmonary function tests. Additional animals were sacrificed and tissue, bronchoalveolar lavage (BALF) and blood were collected for histopathological study and analysis of protein expression.
Cytokine Analyses
Plasma and BALF fluids for this study were collected and sent to Eve Technologies (Calgary, Canada) for 32 cytokine and chemokine multiple analyses. Plasma and BALF fluids were run at a 2-fold dilution with PBS at a pH of 7.5. Alterations in protein expression detected with the multiplex analysis were validated using ELISA kits from R&D Systems, Minneapolis, MN.
Pulmonary Function Tests
Airway responsiveness and respiratory mechanics were assessed with a Flexivent (SCIREQ, Montreal, Canada). Following anesthesia, the mouse trachea was surgically exposed and a cannula was inserted into the trachea and tied off with surgical string. The mouse was then paralyzed with intraperitoneal (IP) injection of 4 mg/kg pancuronium bromide and then connected to a small animal ventilator with a respiratory rate set at 150 breaths/min, tidal volume of 10 ml/kg body weight, and 2.5 cm H2O positive end expiratory pressure (PEEP) for 5 min with 0, 0.5, 1.0, 2.0, and 2.5 microgram/g body weight methacholine delivered sequentially via a nebulizer. After each dose, the Flexivent system would automatically perform a force oscillation cycle and acquire various lung mechanical data including dynamic resistance, elastance, and compliance of the respiratory system. Each methacholine dose delivery and its measurement were completed within a 3 min time frame. The mouse was under continuous thermal support using a heat lamp throughout the pulmonary function test protocol.
Lung Immunohistochemistry and Morphometry
Immunohistochemistry was utilized to localize alpha smooth muscle actin (AB 5694, Abcam,), SNAIL 1 (SC28199, Santa Cruz) TWIST (SC15393, Santa Cruz), ZEB2 (SC4878, Santa Cruz) and NOX4 (AB133303, Abcam). Lungs were fixed by intratracheal instillation of buffered formalin at a constant pressure of 20 cm H2O. Paraffin embedded sections (5 μm) were baked for 1 hour at 65 degrees C then subjected to a series of de-paraffinization (3 × 5 min in xylene) and dehydration. Microwave antigen retrieval method was performed by boiling in 10mM sodium citrate buffer, pH of 6, for 10 min, then cooled for 20 min after the slide was washed with PBS. The slide was then incubated in 1% H2O2 to quench peroxidase activity for 10 min, washed with PBS, and then incubated in 2% normal secondary host serum for 30 min. The slides were incubated with primary antibodies diluted in PBS 0.05% Tween 20 for one hour at room temperature. The antibody dilutions utilized were: 1:100 for SNAIL1, TWIST, and ZEB2 from Santa Cruz. 1:500 for, Alpha SMA and NOX4 at 1:500 from Abcam. After three washes of 3 min each of buffer (PBS with 0.1% Tween 20), the slides were incubated with biotinylated HRP secondary antibodies for one hour using ABC Vector Elite kits (Vector Laboratories, Burlingame, CA). The slides were again washed 3 times for 3 min each wash, incubated with Avidin-Biotin complex for 30 min, and finally administered another 3 washes with buffer solution. The color development was done using a Vector VIP detection solution following the manufacturer’s instructions. Airway collagen was stained using pico-sirius red dye special stain and visualized by polarized light microscopy.
The amount of smooth muscle and collagen around the airways and the expression of SNAIL 1, TWIST, ZEB2 and NOX4 in airway epithelium was quantitated by analyzing digital images collected by using PCI software (Pittsburgh, PA) as previously described (Lantz et al,, 2009). Sections of lung tissue were scanned and all airways cut in cross section (the ratio of maximum to minimum diameter was less than 2) were analyzed. Diameters were determined by filling of the area inside of the airway epithelium. PCI was able to obtain the minimum and maximum diameters of a region of interest. Basement membrane perimeter was obtained by tracing. The area of protein expression was determined by thresholding the images to detect only the antibody staining and measuring the number of pixels detected (Camateros et al, 2007). Area of collagen staining was obtained using polarized light microscopy of sirius red stained sections (Lantz et al, 2009). Area of protein expression and collagen staining were then normalized to the square of the basement membrane perimeter (Camateros et al, 2007). Data were analyzed for all airways and also were subdivided by airway diameter (small airways, diameter < 100 μm versus large airways, diameter > 100 microns). The minimum diameter was used as a measure of the airway diameter (Weibel 1979).
Statistical Analyses
Statistical analyses were performed using ANOVA, requiring p < 0.05 for statistical significance (Winer et al, 1991). Student-Newman-Keuls procedure was used post hoc to determine significant differences between groups.
Results
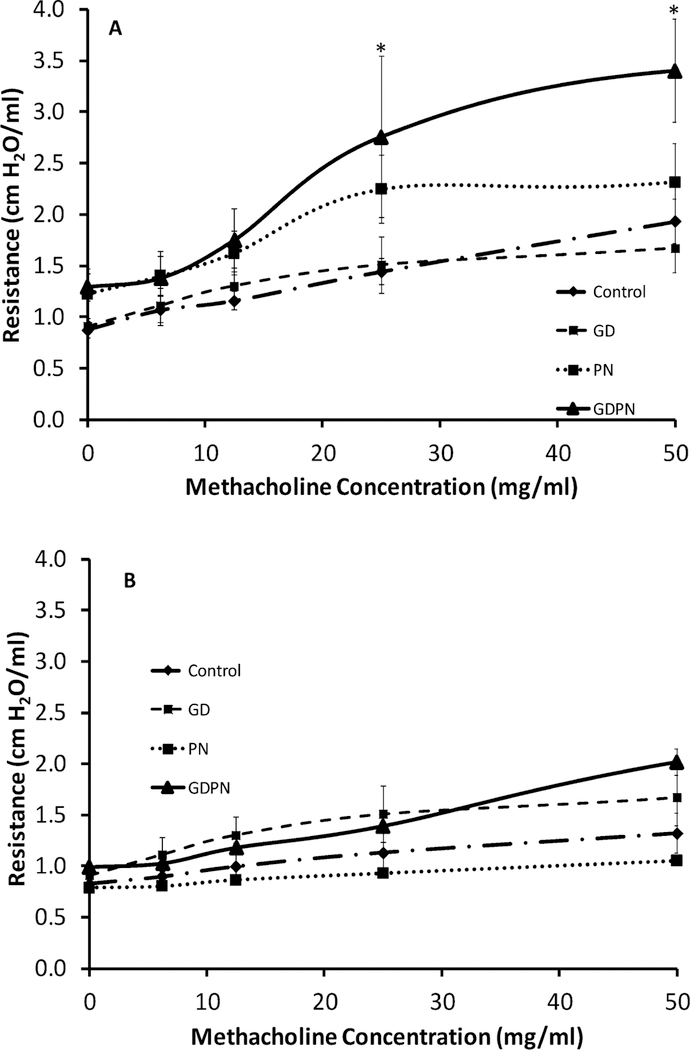
Inhalation exposure of dust from the Iron King and Humboldt Superfund site (IKMSS) in Arizona caused physiological changes in the lungs of male mice. Baseline and methacholine challenged (airway reactivity) values of pulmonary resistance were measured in male (Figure 1A) and female (Figure 1B) mice.
Figure 1.
Airway reactivity following methacholine challenge. 28-day old male (Figure 1A) and female (Figure 1B) mice were assessed for their baseline and methacholine challenged pulmonary resistance. Animals were exposed via inhalation to Iron King Mine tailing dust either in utero (GD) (N=6for males, N=4 for females) only, postnatally only (PN) (N=10 for males, N=4 for females) or during both developmental periods (GDPN) (N=5 for males, N=4 for females). Control animals (N=19 for males, N=7 for females) were exposed to filtered air only. In males, continuous exposure to the dusts throughout development (GDPN) led to significant increases in airway reactivity compared to controls. Females did not show any increase in airway reactivity for any of the exposure groups. * = significantly different from control animals (p<0.05).
Male mice that were exposed either only in utero (GD) or only postnatally (PN) did not show significant increases in methacholine-induced resistance compared to control animals. However, the combined continuous exposure to the dust (GDPN) resulted in a significant increase in airway resistance at lower methacholine challenges when compared to controls. Airway baseline resistance was also significantly increased in the GDPN group in male mice (1.29 +/− 0.13 cm H2O/ml) versus controls (0.89 +/− 0.08 cm H2O/ml). Baseline resistance in the GD and PN groups was not significantly different from controls. There were no significant differences in compliance between controls and any of the three exposure groups. Female mice did not show a similar response. Females did not have a significant increase in the airway reactivity for any of the exposure groups (Figure 1B). Baseline resistance was also not different from controls in the females. Since the females did not demonstrate any pulmonary function alterations, subsequent data were only collected in the males.
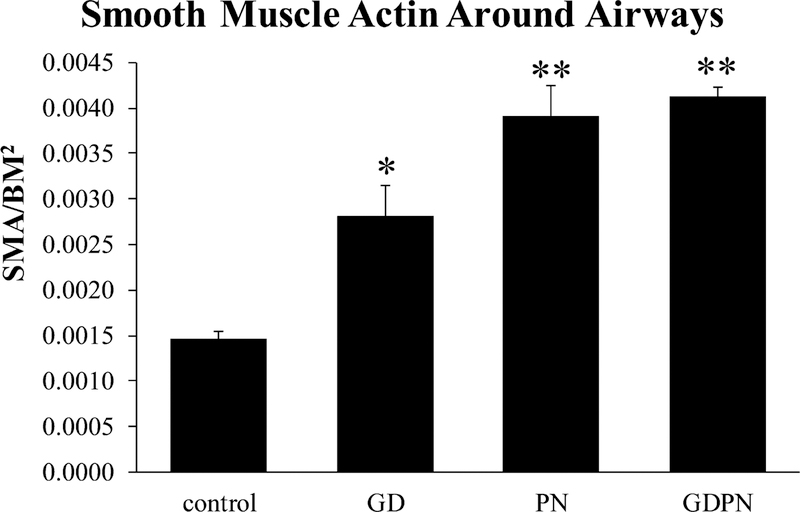
Increases in airway reactivity and baseline resistance seen in the GDPN exposure group could be due to interactions with developmental processes leading to structural alterations to the airways. We have previously shown that continuous in utero and postnatal exposure to arsenic in drinking water resulted in increased airway reactivity associated with alterations in smooth muscle and extracellular matrix around airways (Lantz et al, 2009). These alterations could result from induction in epithelial to mesenchymal transition (EMT) leading to alterations in developmental programing. Alterations in EMT can result in altered smooth muscle and extracellular matrix. We first examined smooth muscle actin levels around airways in 28-day-old male mice to detect airway remodeling in response to dust exposures. All dust exposure groups showed significantly increased smooth muscle actin levels compared to ambient air-exposed controls (Figure 2).
Figure 2.
Smooth muscle actin around airways. All exposure paradigms resulted in significant increases in smooth muscle actin around airways in 28-day old male mice compared to controls (N=4). In addition, PN and GDPN exposed animals had increased smooth muscle compared to in utero alone. GD = in utero only exposure (N=4); PN = postnatal only exposure (N=4); GDPN = both in utero and postnatal exposure (N=3). * = significantly different from controls (p<0.05); ** = significantly different from controls and in utero alone exposures. (p<0.05).
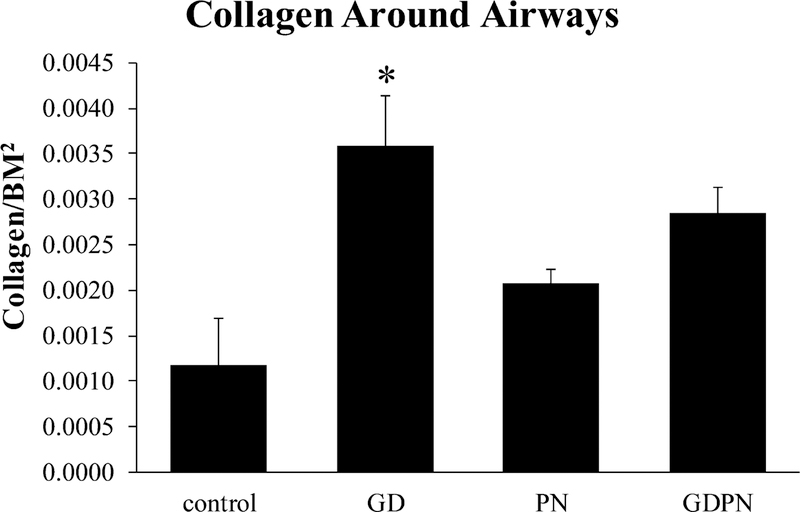
Additionally, the PN and GDPN exposure groups also had increased smooth muscle actin levels when compared to the GD group. Collagen levels around airways in adult mice were only significantly increased in larger (> 100 μm in diameter) airways in the GD group (Figure 3).
Figure 3.
Collagen expression around airways. Increases in airway collagen expression in 28-day old male mice was only significantly increased in large airways (>100 μm) from animals that had only been exposed in utero alone. Controls (N=4); GD = in utero only exposure (N=5); PN = postnatal only exposure (N=6); GDPN = both in utero and postnatal exposure (N=5). * = significantly different from controls (p<0.05)
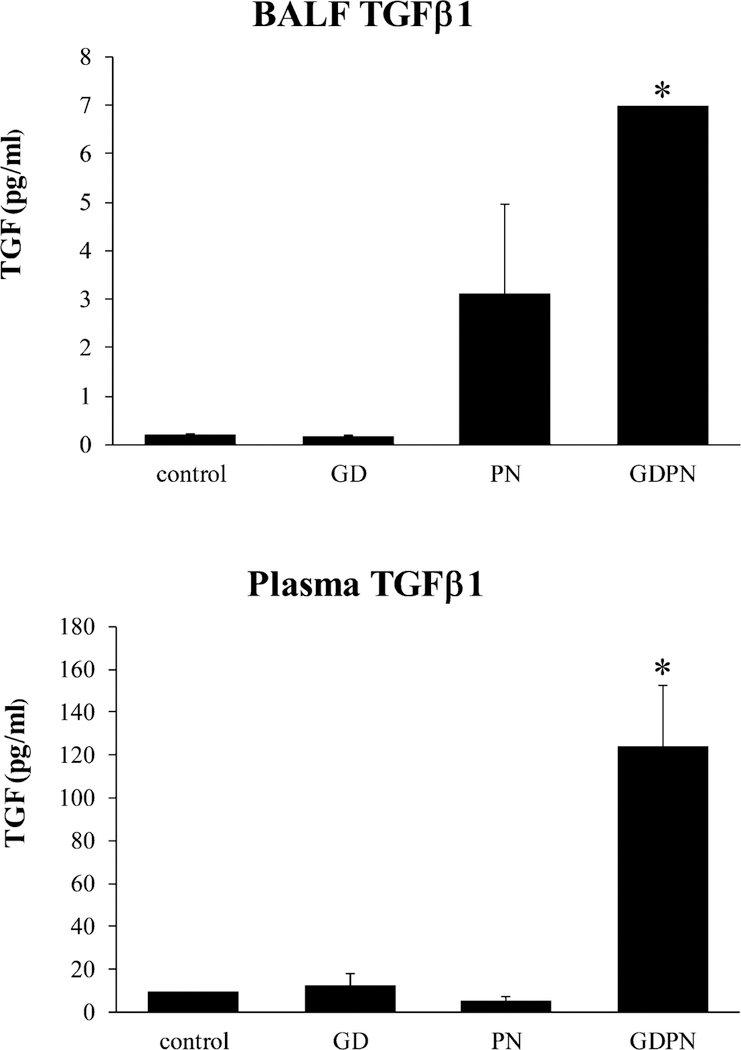
It is accepted that EMT plays a critical role in airway remodeling, (Lamouille et al, 2014; Xu et al, 2009) and that TGFβ1 is a master regulator of EMT. (Mei and Saiz 2014). To determine if dust exposure induced changes in TGFβ1, we evaluated bronchoalveolar lavage fluid (BALF) and plasma (Figure 4) from male mice. We detected significant increases in TGFβ1 in both BALF and plasma in the GDPN exposure group (Figure 4).
Figure 4.
TGFβ1 protein expression in 28-day old male mice following developmental exposure to Iron King Mine tailing dust. Both lung BALF and plasma TGFβ1 levels are significantly increased in the animals that were continuously exposed during development (GDPN). Controls (N=3); GD = in utero only exposure (N=3); PN = postnatal only exposure (N=3); GDPN = both in utero and postnatal exposure (N=3). * = significantly different compared to all other groups (p<0.05)
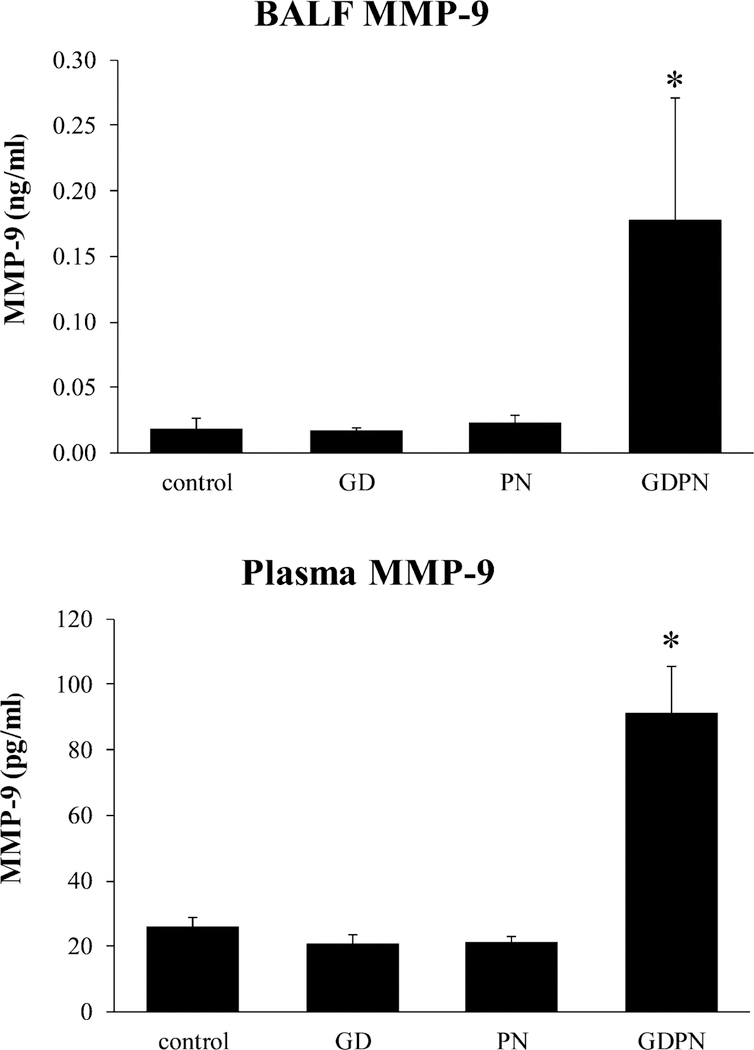
Airway remodeling also involves proteins of the matrix metalloproteinase family, including MMP-9, essential in the breakdown of extracellular matrix in normal physiological processes. (Lin et al, 2012). Similar to that found for TGFβ1, MMP-9 levels were significantly increased in both the BALF and serum fractions in the GDPN group in male mice (Figure 5). Plasma MMP-9 concentrations were increased approximately 4-fold (85 pg/ml) in the GDPN mouse group compared to all other mouse groups which had a mean plasma MMP-9 level at 20 pg/ml. (Figure 5).
Figure 5.
MMP-9 levels in BALF and plasma. MMP-9 was significantly increased in continuously exposed male mice compared to all other groups. Controls (N=3); GD = in utero only exposure (N=3); PN = postnatal only exposure (N=3); GDPN = both in utero and postnatal exposure (N=3). * = significantly different than all other groups (p<0.05)
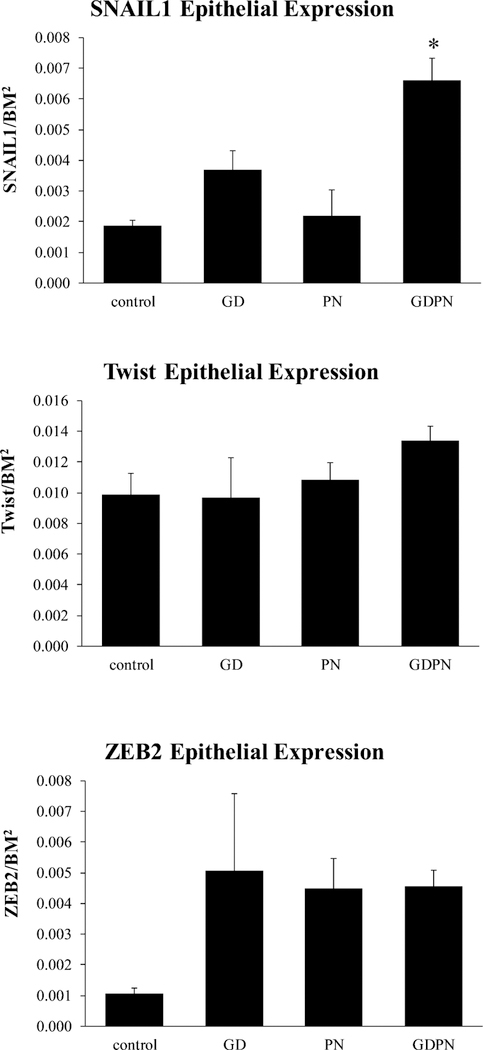
TGFβ1 can regulate EMT through downstream activation of several transcription factors that include SNAIL, TWIST and ZEB. To determine if these transcription factors were influenced by dust exposure and contribute to EMT, we examined their expression in the airway epithelium. SNAIL1 epithelial expression was significantly elevated in the GDPN exposed male mice versus all other groups (Figure 6). While SNAIL 1 levels were significantly elevated following GDPN exposures, TWIST and ZEB 2 levels were not significantly increased over control levels (Figure 6)
Figure 6.
Expression of EMT transcription factors in airway epithelial cells. Continuous developmental (GDPN) exposures caused increased expression of SNAIL1 in male mice compared to all other exposure groups. For SNAIL 1 N=3 for controls, PN and GDPN groups and N=4 for GD group. TWIST and ZEB2 expression were not significantly different from controls. For TWIST, N=3 for all groups. For ZEB2, N=6 for controls, PN and GDPN groups and N=4 for GD group. GD = in utero only exposure; PN = postnatal only exposure; GDPN = both in utero and postnatal exposure. * = significantly different from all other exposure groups. (p<0.05)
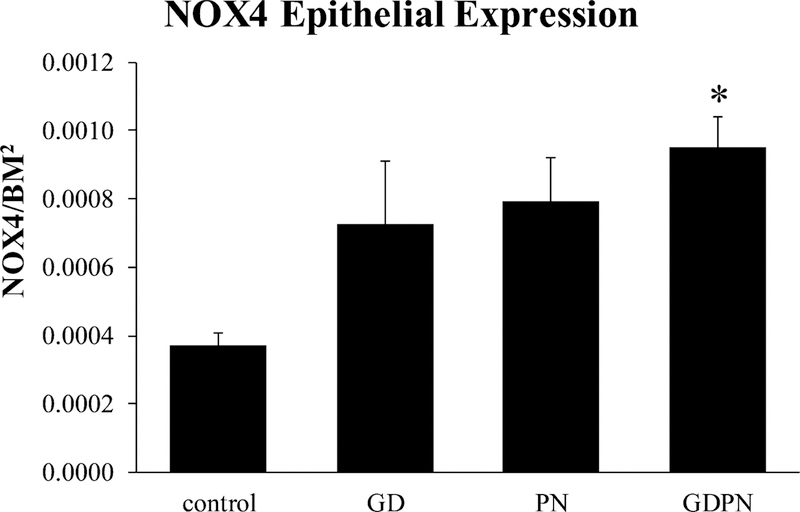
The ability of TGFβ1 to drive EMT depends in part on production of ROS through NADPH oxidase-4 (NOX4) (Boudreau et al, 2012). Although NOX4 is constitutively expressed in endothelial cells, smooth muscle, airway epithelial cells and fibroblasts, overall ROS production varies with NOX4 expression level. TGFβ1 (acting through the SMAD signaling pathway) can increase NOX4 expression, which in turn can upregulate EMT and further increase expression of TGFβ1 in a feed-forward manner. Thus, we examined NOX4 expression in the airway epithelium and found that it was significantly elevated in the GDPN exposed group (Figure 7).
Figure 7.
NOX4 expression in airway epithelial cells. NOX4 was significantly increased versus controls in 28-day old male mice following continuous developmental exposure to IK tailing dust. Controls (N=5); GD = in utero only exposure (N=4); PN = postnatal only exposure (N=4); GDPN = both in utero and postnatal exposure (N=3). * = significantly different from controls (p<0.05)
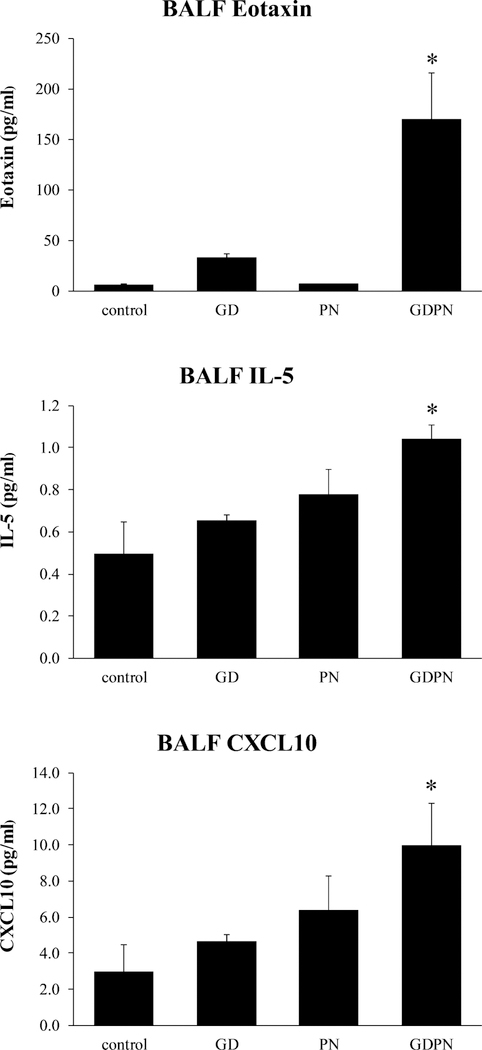
In addition to changes in EMT, increased airway reactivity may be the result of chronic inflammation. We therefore evaluated the effects of early life inhalation exposures to IKMSS mine tailing dust on lung inflammation and cytokine production. Results revealed increased expression in male mice of multiple cytokines associated with eosinophilia in BALF of the GDPN exposure group. These include eotaxin 1, IL-5 and CXCL10. (Figure 8).
Figure 8.
Cytokine expression in BALF. Expression of eosinophil related cytokines in BALF of 28-day old male mice. Continuous in utero and early postnatal exposures resulted in significant increased expression of eotaxin, interleukin-5 and CXCL10. For all groups, N=3; GD = in utero only exposure; PN = postnatal only exposure; GDPN = both in utero and postnatal exposure. * = significantly different from all other groups (p<0.05)
Discussion
Using a mouse model, we have examined the effect(s) of inhalation of real world dusts that occur prior to birth (in utero alone), that occur only after birth and that occurred continuously from before mating, through gestation and birth until the animals reached adulthood (28 days old). Our results indicate that the most significant changes in lung structure and function are observed when exposure occurs in male mice following exposure continuously throughout development. These changes include increased airway hyper-reactivity and baseline resistance, increased expression of epithelial to mesenchymal (EMT) transition protein markers (TGFβ1, MMP-9 and SNAIL1) and increased expression of cytokines related to eosinophils.
Our results for the GDPN exposure group are consistent with the model that inhalation of the real world dusts increases production of ROS by induction of NOX4. This provides a positive feedback to increase TGFβ1 levels and drive EMT leading to alteration in lung structure and function (Boudreau et al, 2012). Our results also show gender differences. Males exposed continuously through gestation and postnatal development had increased airway reactivity, while pulmonary function in females from the same litters was not significantly different from controls. These results are similar to previously reported alterations following early life exposures to PM2.5 (Jedrychowski et al. 2009; Hsu et al. 2015) or ingested arsenic (Raqib et al, 2009).
While the importance of early life exposures to real-world dusts on lung structure and function in adults is recognized, very few reports have addressed this important issue (Mauad et al, 2008; Joad et al, 1995; Tang et al, 2017; Thevenot et al, 2013; Lantz et al, 2009). We believe that this study is the first systematically to quantitate/speciate real-world mine tailings dust and utilize this dust in a mouse model of exposure through inhalation. In the few examples, exposure to real-world airborne toxicants has demonstrated that in utero and early postnatal exposures to PM2.5 can alter lung growth and change adult lung function. Mauad et al, (2008) using an in situ exposure system, located near a heavy traffic street, were able to demonstrate decreased lung function in offspring that had been exposed both in utero and after birth. No differences were observed with either exposure alone. Similarly, Joad et al (1995) showed that both in utero and postnatal exposures to cigarette smoke were required to affect lung function and structure. Our data are in agreement with these studies. The most significant changes in lung structure and function are observed when inhalation exposure to mine tailing dust occurs continuously throughout development. In utero exposures alone did not result in dramatic changes in lung structure and function. The greatest effects required a second exposure after birth. This suggests that in utero exposures primes the lung and alters the response to a postnatal exposure. This could potentially be due to epigenetic changes that occur during in utero exposures. We have not examined epigenetic changes in this study. However, reports in the literature do support this possibility. Available evidence suggests that the modulated activities of DNA methyltransferase (DNMT), histone acetylase (HAT) and histone deacetylase (HDAC) may contribute to the epigenetic changes induced by PM (Wang et al, 2012). Prenatal exposures to PM10 are associated alterations in DNA methylation in children (Breton et al, 2016).
Tang et al, (2017) has shown that maternal exposures to PM constituents can lead to alterations in pulmonary function and EMT in the offspring. In utero exposure to PM2.5 through intraperitoneal injection in the mother leads to altered lung function, increased TGFβ1 production and increased ROS production. While this is not a normal route of exposure for PM, these results do show that maternal exposures to PM constituents can lead to alterations in the offspring. We used the more realistic inhalation route of exposure and did see alterations in lung smooth muscle actin and collagen in the in utero only exposure group. However, other functional and structural parameters were not changed. Differences between our results and those of Tang can be due to differences in route of exposure, effective in utero concentrations and chemical makeup of the PM.
Major metal(oid)s (iron, zinc, arsenic and lead) in the Iron King Mine tailings have been found to be enriched in the PM10 fraction (Gonzales et al, 2014). Because of the complex makeup of the real world dusts we used, we are not able to define which constituents (metals/metalloids) or properties (PM) are associated with the adverse outcomes we have seen. However, responses reported here are similar to those found upon water ingestion of arsenic. Our previous studies and reports by others have shown that continuous in utero and early life arsenic exposures via water ingestion can alter lung phenotype and function (Lantz et al, 2009; Petrick et al, 2009; Smith et al, 2006; Recio-Vega et al, 2014; Smith et al, 2013; Olivas-Calderon et al, 2015). Alterations include increased airway reactivity, increased airway smooth muscle mass (most prominent in smaller airways), increased airway collagen deposition and altered expression of transforming growth factor beta-1 (TGFβ−1) and matrix metalloproteinase-9 (MMP-9). In addition, we have shown an association between levels of an important lung anti-inflammatory protein (club cell protein 16) and soil arsenic in children living close to the Iron King site. This suggests that localized arsenic exposure in the lungs could damage the airway epithelium and predispose children for diminished lung function (Beamer et al 2016). Reports of the effects of early life inhalation exposures to other metals is mostly limited to occupational exposures (welding). Exposures during pregnancy may lead to risk of small-for-gestational-age outcomes (Quansah and Jaakkola 2009). Because of its known developmental neurological effects, of particular concern is potential exposure to lead. However, assessment of children living near the Iron King tailings did not show any increased blood lead levels (Loh et al, 2016).
In addition to epigenetic changes, in utero priming may be a result of immunological changes in the lung. Ferrini et al, (2017) investigated the immunological consequences of prenatal exposure to environmental tobacco smoke (ETS) in order to understand events responsible for the development or exacerbation of allergic asthma. In utero exposure to ETS significantly exacerbated airway eosinophilic inflammation, airway hyperreactivity, mucus secretion, cysteinyl leukotriene biosynthesis, and type 2 cytokine production in the offspring which was dependent on NOX. In our study, NOX4 was significantly elevated in the GDPN mouse group, suggesting ROS from NOX4 may contribute to induction of EMT as shown by Boudreau et al, (2012).
A key inflammatory cell in the development of asthma/airway hyperreactivity may be the eosinophil. Eosinophilia has long been associated with airway hyperreactivity and eosinophils have also been shown to promote EMT through a TGFβ1 mechanism (Yasukawa et al 2013.). Eotaxin 1 is a C-C motif chemokine 11 also known as eosinophil chemotactic protein that selectively recruits eosinophils by inducing their chemotaxis, and therefore, is implicated in allergic responses (Adar et al, (2014). We also measured IL-5, and CXCL10 in this study. IL-5 receptors and IL-5 are expressed by eosinophils and contributes to allergic asthma. (Broughton et al, 2012). CXCL10 (aka, interferon gamma-induced protein 10 (IP-10)) contributes to airway hyperreactivity and eosinophilia. Our chemical mediator data demonstrates that eotaxin, IL-5 and CXCL10 concentrations were only elevated in the GDPN mouse group (Figure 8) consistent with the changes in airway hyperreactivity observed in (Figure 1A).
In addition to increasing airway reactivity, eosinophils can also interact and exacerbate EMT. Yasukawa et al, (2013) induced EMT in bronchial epithelial cells co-cultured with human eosinophils and it was associated with increased expression of TGFβ1 and Smad3 phosphorylation in the bronchial epithelial cells. Treatment with anti-TGFβ1 antibody blocked EMT development in the bronchial epithelial cells.
Arizona has an estimated 100,000 abandoned mines, indicating the potential magnitude of exposure to metal(oid) containing dusts (Arizona State Mine Inspector, 2018). This is not only an issue in the arid southwest of the US but is an issue in arid and semi-arid locations throughout the world where hard rock mining occurs. An assessment of the properties and constituents of the particulate matter that cause adverse health outcomes is important. Our research group has studied the bioaccessibility of mine tailings dust elements from the IKMSS in Arizona. (Thomas et al, 2018). Other researchers have begun to assess the potential toxicity of mine tailings. Martin et al, (2017) used a biologically relevant extract to assess the 24-hour lung bioaccessibility of arsenic in simulated lung lining fluid in dust isolated from four distinct types of historical gold mine wastes common to regional Victoria, Australia. Drahota et al, 2017 studied mine wastes from the historical mining activities in the village of Kank (in the northern part of the Kutna Hora ore district, Czech Republic) which contain significant amounts of metal(oid) contaminants such as arsenic (As), copper (Cu), Pb, and zinc (Zn). Bulk chemical analyses indicated that As was the most important contaminant in the mine wastes (1.15 wt%), urban soils (~ 2900 mg/kg), and road dusts (~ 440 mg/kg) and was found to be the element posing the greatest risk.
A key question in these studies of dust exposure and specifically mine tailings dust exposure is exposure and health outcomes in human populations. Herrera et al, (2016) studied a community in northern Chile that suggested a considerable increase in the risk for respiratory diseases closer to the two mines, and only beyond a minimum distance of more than 1800 meters, the health impact of the mine dusts was considered to be negligible. Our research group found soil As concentrations in the town of Dewey-Humboldt, AZ near the IKMSS to have a significant association with urinary levels of CC16 in children (Beamer et al, 2016). Both arsenic exposure and decreased levels of CC16 in childhood have been associated with decreased adult lung function (Beamer et al, 2016; Bernard et al, 1994; Burgess et al, 2003; Lantz et al, 2009; Parvez et al, 2010; Smith et al, 2013). CC16 appears to be a key protecting protein that is reduced in response to inhaled toxicants
Future Directions
Our data indicate that the most significant changes in lung structure and function are observed when exposure occurs continuously throughout development. Our data suggest that in utero exposures primes the lung for subsequent alterations following additional postnatal exposures. We do not yet know the exact mechanism(s) of the in utero priming. In addition, the role of arsenic and other constituents and properties of the particulate matter that confer developmental alterations in the lung need to be determined. More extensive studies involving children exposed to real world dusts needs to be initiated. These areas should be the focus of future research.
Acknowledgements
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences, [P30ES00694, P42ES004940, R01ES027013]. The Cellular Imaging Core of the Southwest Environmental Health Science Center, managed by Doug Cromey, was essential for assisting us with the capture and analysis of tissue images.
Abbreviations:
- GD
gestational exposure only
- PN
postnatal exposure only
- GDPN
continuous gestational and postnatal exposures
- EMT
epithelial to mesenchymal transition
- IKMSS
Iron King Mine and Humboldt Smelter Superfund Site
References
- Adar T, Shteingart S, Ben Ya’acov A, Bar-Gil Shitrit A, Goldin E 2014. From airway inflammation to inflammatory bowel disease: Eotaxin-1, a key regulator of intestinal inflammation. Clin. Immunol 153:199–208. doi: 10.1016/j.clim2014.04.012 [DOI] [PubMed] [Google Scholar]
- Arizona State Mine Inspector, https://asmi.az.gov/how-many-mines Accessed December 27, 2018
- Beamer PI, Klimecki W, Loh M, Van Horne YO, Sugeng A, Lothrop N, Billheimer D, Guerra S, Lantz RC, Martinez FD. 2016. Association of children’s urinary CC16 levels with arsenic concentrations in multiple environmental media. Int. J. Environ. Res. Public Health 13:521–536. doi: 10.3390/ijerph13050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard AM, Roels HA, Buchet J, Lauwerys RR. 1994. Serum Clara cell protein: An indicator of bronchial cell dysfunction caused by tobacco smoking. Environ. Res 66:96–104. doi: 10.1006/enrs.1994.1047 [DOI] [PubMed] [Google Scholar]
- Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. 2012. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53:1489–1499. doi: 10.1016/freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breshears DD, Kirchner TB,Whicker JJ, Field JP, Allen CD. Modeling aeolian transport in response to succession, disturbance and future climate: Dynamic long-term risk assessment for contaminant redistribution. Aeolian Res 2012;3:445–57. 10.1016/j.aeolia.2011.03.012 [DOI] [Google Scholar]
- Breton CV, Yao J, Millstein J, Gao L, Siegmund KD, Mack W, Whitfield-Maxwell L, Lurmann F, Hodis H, Avol E, Gilliland FD. 2016. Prenatal air pollution exposures, DNA methyl transferase genotypes, and associations with newborn LINE1 and Alu methylation and childhood blood pressure and carotid intima-media thickness in the Children’s Health Study. Enviro. Health Perspect 124:1905–1912. doi: 10.1289/EHP181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. 2012. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immmunol. Rev 250:277–302. doi: 10.1111/j.1600-065X.2012.01164.x. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Witten ML, Nanson CJ, Hysong TA, Sherrill DL, Quan SF, Gerkin R, Bernard AM. 2003. Serum pneumoproteins: A cross-sectional comparison of firefighters and police. Am. J. Ind. Med 44:246–253. doi: 10.1002/ajim.10269 [DOI] [PubMed] [Google Scholar]
- Camateros P, Tamaoka M, Hassan M, Marino R, Moisan J, Marion D, Guiot MC, Martin JG. 2007. Chronic asthma-induced airway remodeling is prevented by toll-like receptor-7/8 ligand S28463. Am. J. Respir. Crit. Care Med 175:1241–1249. doi: 10.1164/rccm.200701-054OC [DOI] [PubMed] [Google Scholar]
- Chan JK, Fanucchi MV, Anderson DS, Abid AD, Wallis CD, Dickinson DA, Kumfer BM, Kennedy IM, Wexler AS, Van Winkle LS. 2011. Susceptibility to inhaled flame-generated ultrafine soot in neonatal and adult rat lungs 124:472–86. doi:1093/toxsci/kfr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. 2010. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect 118:284–290. doi: 10.1289/ehp.0900916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Landazuri A, Wonaschutz A, Rine K, Rheinheimer P, Barbaris B, Conant W, Saez AE, Betterton EA. 2011. Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water Air Soil Pollut 221: 145–157. doi: 10.1007/s11270-011-077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahota P, Raus K, Rychlikova E, Rohovec J. 2017. Bioaccessibility of As, Cu, Pb, and Zn in mine waste, urban soil, and road dust in the historical mining village of Kank, Czech Republic. Environ. Geochem. Health 39:1–18. doi: 10.1007/s10653-017-9999-1 [DOI] [PubMed] [Google Scholar]
- Farzan SF, Karagas MR, Chen Y. 2013. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272: 384–390. doi: 10.1016/j.taap.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. 2008. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 38: 57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini M, Carvalha S, Cho YH, Postma B, Miranda Marques L, Pinkerton K, Roberts K, Jaffar Z. 2017. Prenatal tobacco smoke exposure predisposes offspring mice to exacerbated allergic airway inflammation associated with altered innate effector function. Part. Fibre Toxicol 14:30. doi: 10.1186/s12989-017-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales P, Felix O, Alexander C, Lutz E, Ela W, Saez AE. 2014. Laboratory dust generation and size-dependent characterization of metal and metalloid-contaminated mine tailings deposits. J. Haz. Materials 280:619–626. doi: 10.1016/j.hazmat.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Herrera R, Radon K, von Ehrenstein OS, Cifuentes S, Munoz DM, Berger U. 2016. Proximity to mining industry and respiratory diseases in children in a community in northern Chile: A cross-sectional study. Environ. Health 15:66–76. doi: 10.1186/s12940-016-014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H-HL, Chiu Y-HM, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ. 2015. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med 192:1052–1059. doi: 10.1164/rccm.201504-0658OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowskia W, Perera F, Mrozek-Budzyna D, Mroza E, Flak E, Spengler JD, Edwards S, Jacek R, Kaim I, Skolicki Z. 2009. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ Res 109:447–456. doi: 10.1016/j.envres.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joad JP, Ji C, Kott KS, Bric JM, Pinkerton KE. 1995. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol. Appl. Pharmacol 132:63–71. [DOI] [PubMed] [Google Scholar]
- Joad JP, Bric JM, Peake JL, Pinkerton KE. 1999. Perinatal exposure to aged and diluted sidestream cigarette smoke produces airway hyperresponsiveness in older rats. Toxicol. Appl. Pharmacol 155:253–260. doi: org/ 10.1006/taap.1998.8612. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial-mesenchymal transition. Nature Rev./Mol. Cell Biol 15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Chau B, Sarihan P, Witten ML, Pivniouk VI, Chen GJ. 2009. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Tox. Appl. Pharm 235:105–113. doi: 10.1016/j.taap.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Yokoyama H, Rac VE, Brooks SC. 2012. Novel biomarkers in diagnosing cardiac ischemia in the emergency department: A systematic review. Resuscitation 83:684–691. doi: 10.1016/j.resuscitation.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Loh MM, Sugeng A, Lothrop N, Klimecki W, Cox M, Wilkinson ST, Lu Z, Beamer PI. 2016. Multimedia exposures to arsenic and lead for children near an inactive mine tailings and smelter site. Environ Res 146:331–339. doi: 10.1016/j.envres.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Dowling K. Nankervis S, Pearce D, Florentine S, McKnight S. 2017. In vitro assessment of arsenic mobility in historical mine waste dust using simulated lung fluid. Environ. Geochem. Health 39:1–13. doi: 10.1007/s10653-016-9833-1. [DOI] [PubMed] [Google Scholar]
- Mauad T, Rivero DH, de Oliveira RC, Lichtenfels AJ, Guimaraes ET, deAndre PA, Kasahara DI, Bueno HM, Saldiva PH. 2008. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am. J. Respir. Crit. Care Med 178:721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar KK, Guha Mazumder DN. 2012. Effect of drinking arsenic contaminated water in children. Indian Pediatr 56:223–226. doi: 10.4103/0019-557X.104250 [DOI] [PubMed] [Google Scholar]
- Mei Q and Saiz L. 2014. Literature-based automated reconstruction, expansion, and refinement of the TGF-beta superfamily ligand-receptor network. J. Membr. Biol 247:381–386. doi: 10.1007./s00232-014-9643-2. [DOI] [PubMed] [Google Scholar]
- Merkus PJ. 2003. Effects of childhood respiratory disease on the anatomical and functional development of the respiratory system. Paed. Respir. Rev 4:28–39. [DOI] [PubMed] [Google Scholar]
- Olivas-Calderon E, Recio-Vega R, Gandolfi AJ, Lantz RC, Gonzalez-Cortes T, Gonzalez-De Alba C, Froines JR, Espinosa-Fematt JA. 2015. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicology Applied Pharmacol 287:161–167. doi: 10.1016/j.taap.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, Argos M, Hassan R, Yunus M, Haque SE, et al. , 2010. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax 65:528–533. doi: 10.1136/thx.2009.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Blachere FM, Lantz RC. 2009. Inorganic arsenic as a developmental toxicant: in utero exposure and alterations in the developing rat lungs. Mol. Nutr. Food Res 53:583–591. doi:10.1002.mnfr.200800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quansah R, Jaakkola JJK. 2009. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Inter Archives Occupational Environ Health 82:529–537. doi 10.1007/s00420-008-0349-6 [DOI] [PubMed] [Google Scholar]
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, Nermell B, Yunus M, Roy S, Persson LA, Arifeen SE, Moore S, Vahter M. 2009. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett 185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Alba CG. 2014. In utero and early childhood exposure to arsenic decreases lung function in children. J. Appl. Tox doi: 10.1002/jat.3023. [DOI] [PMC free article] [PubMed]
- Shi W, Bellusci S, Warburton D. 2007. Lung development and adult lung diseases. Chest 132: 651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. 2006. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect 114: 1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Yunus M, Khan AL, Ercumen A, Yuan Y, Smith MH, Liaw J, Balmes J, Ehrenstein OV, Raqib R, et al. , 2013. Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int. J. Epidemiol 42:1077–1086. doi: 10.1093/ije/dyt120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann K, Snape N, Baturcam E, Fantino E. 2016. The impact of early-life exposure to airborne environmental insults on the function of the airway epithelium in asthma. Annals Global Health 82:2214–2227. 10.1016/j.aogh.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Stovern M, Guzman H, Rine KP, Felix O, King M, Ela WP, Betterton EA, Saez E. 2016. Windblown dust deposition forecasting and spread of contamination around mine tailings. Atmosphere (Basel) doi: 10.3390/atmos 7020016. [DOI] [PMC free article] [PubMed]
- Tang W, Du L, Sun W, Zhiqiang Y, Fang H, Chen J, Li X, Li X, Yu L, Chen D. 2017. Maternal exposure to fine particulate air pollution induces epithelial-to-mesenchymal transition resulting in postnatal pulmonary dysfunction mediated by transforming growth factor-beta/Smad3 signaling. Toxicol. Letters 267:11–20. doi: 10.1016/j.toxlet.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Thevenot PT, Saravia J, Jin N, Giaimo JD, Chustz RE, Mahne S, Kelley MA, Hebert VY, Dellinger B, Dugas TR, et al. , 2013. Radical-containing ultrafine particulate matter initiates epithelial-to-mesenchymal transitions in airway epithelial cells. Am. J. Respir. Cell Mol. Biol 48:188–197. doi:10.1165.rcmb.2012-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AN, Root RA, Lantz C, Saez E, Chorover J. 2018. Oxidative weathering decreases bioaccessibility of toxic metal(loid)s in PM10 emissions from sulfide mine tailings. Geohealth 2:118–138. doi: 10.1002/2017GH000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M 2008. Health effects of early life exposure to arsenic. Basic Clin. Pharmacol. Toxicol 102:204–211. doi:10.1111.j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Garcia JGN, Zhang W. 2012. Epigenetic regulation in particulate matter-mediated cardiopulmonary toxicities: a systems biology perspective. Curr Pharmacogenomics Person Med 10: 314–321. doi: 10.2174/187569212803901792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Bellusci S, Warburton D 2007. Lung development and adult lung diseases. Chest 132:651–656. doi: 10.1378/chest.06-2663 [DOI] [PubMed] [Google Scholar]
- Weibel ER. 1979. Stereological Methods, Volume 1: Practical Methods for Biological Morphometry Academic Press, London. [Google Scholar]
- Whitsett JA, Haitchi HM, Maeda Y. 2011. Intersections between pulmonary development and disease. Am. J. Respir. Crit. Care Med 184:401–406. doi: 10.1164/rccm.201103-0495PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design McGraw-Hill, New York, 1991. [Google Scholar]
- Xu J, Lamouille S, Derynck R. 2009. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19:156–172. doi: 10.1038/cr.2009.5.Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa A, Hosoki K, Toda M, Miyake Y, Matsushima Y, Matsumoto T, Boveda-Ruiz D, Gil-Bernabe P, Nagao M, Sugimoto M, Hiraguchi Y, Tokuda R, Naito M, Takagi T, D’Alessandro-Gabazza CN, Suga S, 3 Kobayashi T, Fujisawa T, Taguchi O, Gabazza EC. 2013. Eosinophils Promote Epithelial to Mesenchymal Transition of Bronchial Epithelial Cells. PLoS One 2013; 8(5): e64281. doi: 10.1371/journal,pone.0064281 [DOI] [PMC free article] [PubMed] [Google Scholar]