Abstract
Continuous femoral nerve block (CFNB) has been used to prevent the breakthrough pain after total knee arthroplasty (TKA). Multimodal drug injection (PMDI) has also been shown to decrease opioid consumption and pain. We investigated whether the use of PMDI further improves analgesic and rehabilitation outcomes when used in conjunction with CFNB. This is a prospective randomized controlled study of 44 patients undergoing primary TKA. The treatment group (n = 23) received a PMDI of combined ropivacaine, epinephrine, ketorolac and morphine, and the controlled group (n = 21) received saline at wound closure. Total opioid consumption, pain scores, knee range of motion (ROM) outcomes, length of stay, and patient satisfaction were measured and compared. The total consumption of morphine is similar between the two groups (52.6 ± 40.6 vs. 41.5 ± 32.9, p = 0.325). The mean morphine consumption of the treatment group was significantly lower than the control at 4 h after surgery (4.2 ± 5.5 vs. 11.3 ± 8.1, p = 0.002) but comparable on POD1, POD2, and POD3. The mean pain scores were significantly higher in the treatment group than the control group at POD2 (at rest: 47.3 ± 29.1 vs. 23.8 ± 20.6, p = 0.004; after PT: 57.7 ± 25.4 vs. 35.2 ± 26.8, p = 0.007) and POD3 (at rest: 30.9 ± 30.3 vs. 14.8 ± 20.9, p = 0.045; after PT: 50.2 ± 30.6 vs. 29.0 ± 32.1, p = 0.035), and not significantly different at 4 h after surgery or at POD1. Mean maximal knee flexion ROM in degrees during active and active assisted mobilization showed no significant difference between the control and the treatment groups on POD2 and POD3. The mean length of stay of the treatment group is significantly longer than the control group (5.1 ± 2.1 vs. 3.8 ± 1.6, p = 0.032). At discharge, no significant difference exists between the two groups for mean patient satisfaction. The addition of PMDI led to a decrease in opioid consumption in the immediate postoperative period but with no significant difference in the total consumption within the first three days postoperatively. This finding provides an opportunity for appropriate preoperative treatment and education for both patients and caregivers.
Keywords: Periarticular injection, Total knee arthroplasty, Perioperative pain control
1. Introduction
Effective pain management is crucial for successful outcomes and patient satisfaction following total knee arthroplasty (TKA).1, 2 Proper analgesia can not only facilitate early rehabilitation and reduce hospital length of stay, but can even result in decreased healthcare costs.3 The administration of parenteral opioids including patient controlled analgesia (IVPCA) have been historically utilized as the mainstay treatment for acute postoperative pain. However, opioids may not always provide adequate pain relief, and are commonly associated with undesirable side effects, such as sedation, nausea, vomiting, ileus, urinary retention, hypotension, pruritus, and respiratory depression.4, 5, 6 In order to maximize the analgesia and to minimize the side effects, a multimodal approach with preemptive analgesia has become popular, and includes a combination of peripheral nerve blocks, epidural catheters, non-steroidal anti-inflammatory drugs (NSAIDs), oral narcotics, and periarticular multimodal drug injection (PMDI).7, 8, 9, 10
Continuous femoral nerve blocks (CFNB) are known to improve analgesia after TKA when used in conjunction with a multimodal approach.11, 12, 13 However, some patients may still experience breakthrough pain, possibly due to the fact that the obturator and sciatic nerve distributions are not blocked. Similarly, a PMDI of ropivacaine, ketorolac and morphine has shown to decrease opioid consumption and postoperative pain after TKA.14, 15, 16 This drug combination has been shown to be synergistic; and the addition of epinephrine has shown to reduce the potential toxicity of the ropivacaine by reducing its systemic distribution.7 Both CFNB and PMDI have been shown to improve pain independently after a primary TKA.17 We hypothesized that a combination of PMDI and a CFNB further reduced opioid consumption and postoperative pain in patients undergoing TKA. In addition, we attempted to determine if patients with this pain control regimen experience better early rehabilitations, early discharge and have less opioid related side effects than those who did not.
2. Materials and methods
After obtaining approval by our Institutional Review Board (IRB), we conducted a single center, prospective, placebo-controlled, double blind trial to assess the efficacy of PMDI of a multi-drug regimen in addition to a CFNB in alleviating pain after TKA when used in conjunction with an established multimodal regimen (Table 1). All patients scheduled for elective unilateral primary TKA for a primary osteoarthritis between the years April 2009-July 2013 at an academic teaching hospital were considered for this study. Patient exclusion criteria included: (1) age younger than 18 years, (2) weight exceeding 120 kg, (3) inability to understand pain scales or the use of the IVPCA device, (4) history of chronic opioid consumption, (5) opioid use in last 6 weeks before surgery, (6) chronic pain syndromes, (7) allergy to local anesthetics and/or opioids, (8) previous lower extremity vascular surgery, (9) peripheral neuropathy, (10) diagnosis other than primary osteoarthritis, and (11) unwilling to participate in the study.
Table 1.
Total joints protocol.
| Pre-op (holding area) meds |
| Coumadin 5 mg PO × 1 dose (ONLY in select pts) |
| Protonix 40 mg PO × 1dose |
| Oxycontin 10 mg PO × 1dose (avoid in elderly and pts with sleep apnoea) |
| Celebrex 200 mg po × 1 dose (not for sulphur allergic pts) (not available at County) |
| Gabapentin 300 mg |
| Anesthesia |
| Spinal with bupivacaine 0.5% 12–15 mg to attain a standard T10 level block |
| Intra-op Cocktail |
| Treatment group |
| Ropivacaine 400 mg |
| Epinephrine diluted 1:1000 in saline (0.6 mL) |
| Ketorolac 30 mg |
| Morphine sulfate 5 mg |
| *** Diluted to a total volume of 100 mL with normal saline |
| Control group |
| 100 mL normal saline |
| Continuous femoral nerve block |
| Ropivacaine 0.2% infused at 5 mL/h |
| Orthopedic Floor |
| Celecoxib 200 mg orally, daily for 10 days ((not for sulphur allergic pts) |
| Acetaminophen 650–1000 mg orally q 4–6 h (total dose of not to exceed 4 gm/day) |
| Gabapentin 300 mg (caution in renal disease and delirious pts) |
| Ketorolac IM q 6 h PRN |
| 15 mg if more than 65 years, 30 mg if less than 65 years; |
| Hold if renal impairement, GI bleeding or other contraindication |
| Not more than 3 days |
| If ketorolac is ineffective, morphine 2–4 mg IM q 2–4 h prn for pain |
Using a computer-generated list of random numbers, patients were randomized and blinded to receive either a PMDI mixture (treatment group) (Table 1) or a placebo periarticular injection containing 100 mL of normal saline (control group). All patients included in this study received a spinal anesthesia of 12–15 mg of bupivacaine 0.5% to attain a standard T6-T8 level block for the surgery. These surgeries proceeded with a standard medial parapatellar approach under tourniquet and a drain was placed before wound closure. In the treatment group, PMDI containing ropivacaine 400 mg, epinephrine diluted 1:1000 in saline (0.6 mL), ketorolac 30 mg and 5 mg of morphine were diluted to a total volume of 100 mL with normal saline was infiltrated around the knee joint into the posterior aspect of the capsule, medial and lateral collateral ligaments and gutters, and the subcuticular tissues.
Postoperatively, all patients had access to postoperative IVPCA for breakthrough pain in addition to the standard multiple modal pain regimen before and after the procedure (Table 1). In the post-anesthesia care unit (PACU), when the patients show some return of motor function, a CFNB was placed for all subjects. A femoral nerve catheter was introduced by the same anesthesiologist under ultrasound guidance. The femoral artery was visualized and the quadriceps contractions were obtained via a neurostimulator. Femoral nerve was also located in an out-of-plane approach. Following negative aspiration, approximately 15 mL of 0.5% ropivacaine was injected. Ultrasound confirmed the dispersion of the solution around the nerve bundle. A catheter was inserted beyond the tip of the needle without resistance then a continuous infusion filled with ropivacaine 0.2% at 5 mL/h was started via an elastomeric pump. A morphine IVPCA consisting of a non-basal infusion of morphine sulfate (1 mg) every 6 min with 10 mg maximum per hour, was initiated in the PACU and continued until postoperative day 3. Thereafter, patients were transitioned to oral pain medications.
Patient parameters such as gender, age, BMI and ASA score were collected for comparison. Postoperative outcome data were collected by a pain nurse practitioner who was blinded to the randomization assignments. Primary outcome variables collected were opioid consumption and pain scores. Daily opioid consumption was recorded in terms of milligram total morphine used via intravenous PCA. A visual analog scale (VAS) with scores ranging from 0 to 100 was used to capture the pain score at rest four hours after surgery. In addition, pain scores at rest and after physical therapy (PT) on postoperative day (POD) 1, POD 2, and POD 3 were also obtained.
Mobilization was encouraged on the day of surgery and formal PT with weight bearing as tolerated was started on the morning of postoperative day 1 with two sessions each day of hospital stay. Secondary outcome variables included active (self initiated) and active assisted (PT assisted) knee ROM (measured in degrees) on POD 2 and POD 3, medication side effects, length of stay, and patient satisfaction. From POD 1 until discharge, patients performed daily knee flexion and extension exercises. The degree of active and active assisted ROM achieved by the patients was measured by a physical therapist starting POD 2. Side effects from any of the medications and pain modalities, including nausea, vomiting, dizziness, drowsiness, confusion, pruritus, constipation, hypotension, urinary retention, respiratory depression, and fall, were also documented. Patient satisfaction was assessed using a simple satisfaction survey questionnaire administered just before discharge. Patients were asked to quantify their degree of satisfaction using a scale from 0 to 10.
Analysis was done with the aid of a statistical software (IBM, SPSS Version 22, New York, NY). A power analysis was performed based on estimates used in a previous study by Adam et al.,11 which revealed that 20 patients were needed per group, with a maximum a beta-error of 20%, for detecting a 40% difference in morphine consumption at a significance level of 0.05. Descriptive statistical analysis with means and their standard deviation values for measurements at each time point were calculated. Data were analyzed to test the differences in primary measures between the control and the experimental groups using either a Student’s unpaired 2-tailed t-tests or a contingent Mann-Whitney U test on the scale and distributional characteristics of the variables using a p value of less than 0.05 to consider the differences between the cohorts as statistically significant.
3. Results
Forty-four patients enrolled in this study and were allocated into treatment group (n = 23) and control group (n = 21). Demographic characteristics between the two groups were comparable in terms of age (64.76 ± 8.35 control vs. 62.39 ± 11.39 experimental), and BMI (32.83 ± 5.47 control vs. 33.12 ± 5.52 experimental). There are 85.7% females (n = 18) in the control group and 73.9% females in the experimental group (n = 17). The ASA scores for patients from both groups range from II–III with 42.9% (n = 9) of the control and 56.5% (n = 13) of the experimental with ASA score of II, and 57.1% (n = 12) of the control and 43.5% (n = 10) of the experimental with ASA score of III (Table 2).
Table 2.
Demographic information of control and experimental groups.
| Control | Experimental | |
|---|---|---|
| (n = 21) | (n = 23) | |
| Age (yr) | 64.76 ± 8.35 | 62.39 ± 11.39 |
| BMI | 32.83 ± 5.47 | 33.12 ± 5.52 |
| Males%(n) | 14.3(3) | 26.1(6) |
| Females%(n) | 85.7(18) | 73.9(17) |
| ASA II%(n) | 42.9(9) | 56.5(13) |
| ASA III%(n) | 57.1(12) | 43.5(10) |
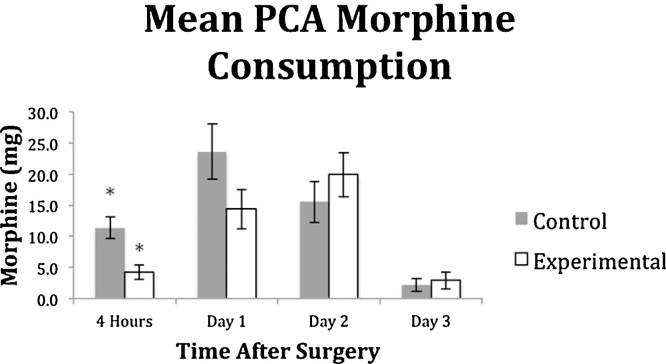
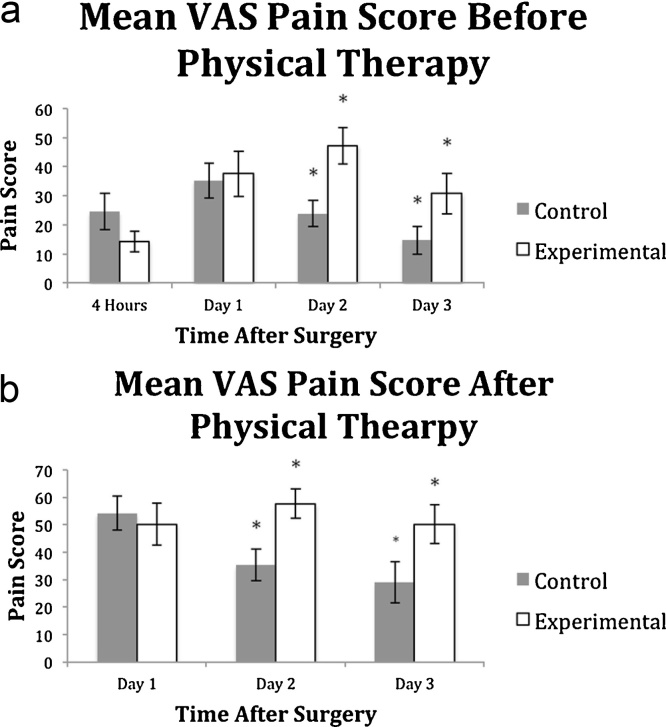
The total consumption of morphine is similar between the two groups (52.6 ± 40.6 vs. 41.5 ± 32.9, p = 0.325). By time period, the mean morphine consumption of the treatment group was significantly lower than the control at 4 h after surgery (4.2 ± 5.5 vs. 11.3 ± 8.1, p = 0.002) but similar on POD1 (14.4 ± 15.0 vs. 23.6 ± 20.4, p = 0.100), POD2 (19.9 ± 17 vs. 15.5 ± 13.6, p = 0.348) and POD3 (2.9 ± 6.4 vs. 2.2 ± 4.1, p = 0.653) (Fig. 1). The mean VAS scores were significantly higher in the treatment group vs the control group at POD2 (at rest: 47.3 ± 29.1 vs. 23.8 ± 20.6, p = 0.004; after PT: 57.7 ± 25.4 vs. 35.2 ± 26.8, p = 0.007) and POD3 (at rest: 30.9 ± 30.3 vs. 14.8 ± 20.9, p = 0.045; after PT: 50.2 ± 30.6 vs. 29.0 ± 32.1, p = 0.035), and not significantly different at 4 h after surgery (14.3 ± 17.0 vs. 24.5 ± 28.9, p = 0.169) or at POD1 at rest and after PT (at rest: 37.6 ± 35.3 vs. 35.2 ± 27.9, p = 0.805; after PT: 50.2 ± 34.8 vs. 54.2 ± 26.7, p = 0.676) (Fig. 2a and b). A standard mixture of PMDI was used in all patients in the treatment group, weight adjusted doses did not show a significant correlation with morphine consumption (r = 0.074, p = 0.570 for all agents, ropivacaine, epinephrine, ketorolac, and morphine sulfate).
Fig. 1.
Mean morphine patient-controlled analgesia (PCA) consumption at sequential times after surgery for control and experimental groups. The bars depict mean values for experimental and control groups, with whiskers representing standard error values. The mean PCA consumption of the treatment group was significantly lower than the control at 4 h after surgery (p = 0.002) but not significantly lower on POD1 (p = 0.100), POD2 (0.348) and POD3 (0.653).
Fig. 2.
a&b Mean pain scores for control and experimental groups before and after physical therapy (PT) using a visual analog scale (VAS) from 0 to 100. Bars depict mean values for experimental and control groups, with whiskers representing standard error values. Before PT (Fig. 2a), the mean pain score was significantly higher in the treatment group at POD2 (p = 0.004) and POD3 (p = 0.045). After PT (Fig. 2b), the mean pain score was significantly higher for the treatment group at POD2 (p = 0.007) and POD3 (p = 0.035).
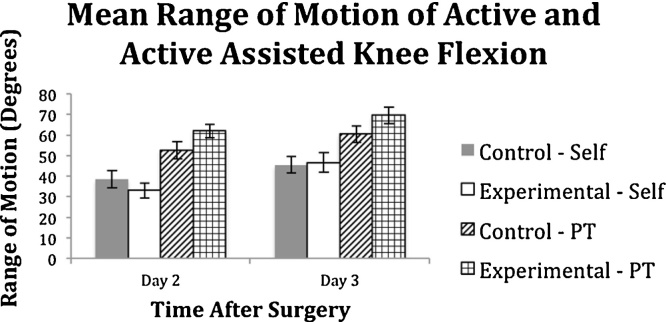
Mean maximal knee flexion ROM in degrees during active and passive knee mobilization were measured on POD2 and POD3 and showed no significant difference between the control and the treatment groups for all conditions on POD2 (active: 33.1 ± 16.3 vs. 38.7 ± 17.5, p = 0.320; assisted active: 62.0 ± 13.8 vs. 52.7 ± 17.5, p = 0.084) and POD3 (active: 46.5 ± 20.4 vs. 45.4 ± 16.7, p = 0.864; assisted active: 69.7 ± 16.8 vs. 60.4 ± 15.6, p = 0.107) (Fig. 3).
Fig. 3.
Mean maximal knee flexion range of motion (ROM) in degrees of control and experimental groups on POD2 and POD3, during active and assisted active (PT assisted) knee mobilization. Bar graph depicts mean values with whiskers representing standard error values. No significant difference between the control and the experimental groups for all conditions on either POD2 or POD3.
There was no significant difference in the opioid side effects’ profiles between the two groups at 4 h after surgery (8.7% vs. 9.52%, p = 0.904), POD1 (34.78% vs. 42.86%, p = 0.779), POD2 (21.74% vs. 14.28%, p = 0.522), and POD3 (52.17% vs. 47.62%, p = 0.764). Side effects experienced by the two groups include constipation, confusion, dizziness, drowsiness, nausea, pruritus, and pruritus. No adverse effect of the PMDI or CFNB such as falls was seen in this study (Table 3). The mean length of stay of the treatment group is significantly longer than the control group (5.1 ± 2.1 vs. 3.8 ± 1.6, p = 0.032) with large percentage of control group being charged by POD3 (66.7% vs. 43.4%, p = 0.124). At discharge, no significant difference exist between the two groups for mean satisfaction scores, control M (SD) = 8.3(1.8), treatment M (SD) = 9.0(1.2).
Table 3.
Opioid side effect profile of each group at different time intervals.
| Control (n = 21) |
Experimental (n = 23) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 h | POD1 | POD2 | POD3 | Any SE | 4 h | POD1 | POD2 | POD3 | Any SE |
| 0 | N/Dz | Dz | Dz | 1 | 0 | 0 | N | 0 | 1 |
| N/V | N/V | N/V/Dz | Dz | 1 | Cf | Cf | Cf | Dz | 1 |
| 0 | Dz | Dz | 0 | 1 | 0 | N/V/Dz | N/V/Dz | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | P/Dz | N/Dz | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | Dz | 0 | 0 | 1 |
| 0 | Dw | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0 | Dz | Dw | 0 | 1 | 0 | Dz | 0 | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | N | 0 | 0 | 1 | 0 | Dz | 0 | 0 | 1 |
| Dw | Dw | Dw | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | Dz | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | C | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | P | 1 | N | 0 | 0 | 0 | 1 |
| 0 | V | 0 | 0 | 1 | 0 | N | 0 | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | Dz | 0 | 1 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | |||||
| 0 | N | 0 | 0 | 1 | |||||
| 9.52% | 42.86% | 23.81% | 14.28% | 47.62% | 8.70% | 34.78% | 21.74% | 8.70% | 52.17% |
Table shows the percentages of control and experimental group subjects experiencing opioid side effects (SE) at each time interval: 4 h, postoperative days (POD) 1, 2 and 3, and total of any SE per group. There were no significant differences between the groups at 4 h even though a significant difference in opioid consumption was seen. Opioid side effects: (C) constipation; (Cf) confusion; (Dz) dizziness; (Dw) drowsiness; (N) nausea; (P) pruritus; (V) vomiting.
4. Discussion
Effective analgesia facilitates early rehabilitation, reduces hospital length of stay, and decreases overall healthcare costs.3 Opioids have been a historical mainstay in perioperative analgesia but have a wide range of undesirable side effects that can affect rehabilitation.4, 5, 6 Periarticular multimodal drug injection, continuous femoral nerve blocks, as well as other multimodal regimens improve analgesia while avoiding the side effects of opioids.7, 8, 11, 12, 13 There has been comparisons of single shot FNB with single shot PMDI, and continuous PMDI to continuous FNB,14 but there is a lack of evidence specifically comparing continuous FNB with and without PMDI. PMDI with FNB in TKA leads to a decrease in opioid consumption within the first four hours after surgery. However, the difference in the total morphine consumption over the first three days postoperatively is not statistically significant.
This study had several limitations. A single standard dose of ropivacaine was used instead of weight-adjusted dose. This was standardized between our two cohorts, but toxic plasma levels were not reached despite high concentration injection as demonstrated in previous studies.18, 19 Second, the residual effects of intraoperative spinal anesthesia, which could vary in different patients, may have confounded the pain score and opioid consumption outcomes during the first 4 h of the postoperative period, but this was same for both groups. Although the study was statistically powered, the number of patients enrolled was relatively smaller with a relatively larger dropout in our setting. The pain and satisfaction scores are often subjective and dependent on individual patient characteristics, and thus are unavoidable sources of bias in any pain management study. Although there are several other options in a multimodal approach, we have only compared the addition of PMDI option to a continuous FNB, and thus this study does not identify the best available option for analgesia after a primary TKA. Despite these limitations, in an adequately powered randomized doubly blinded study, we were able to show that the total consumption of opiate did not differ significantly between the treatment and the control groups.
The current literature is saturated with studies analyzing PMDI and other multimodal regimens. Busch et al.15 compared the use of a PMDI mixture 400 mg of ropivacaine, 30 mg Ketorolac, 5 mg of epimorphine, and 0.6 mL of Epinephrine (1:1000) intraoperative injection with no injections and showed injections significantly reduced patient-controlled analgesia with decreased VAS for pain and increased VAS for satisfaction during first 24 h; however, their evaluation was limited to the first 24 h postoperatively. In our study, we showed significant decrease in opioid consumption in the PMDI group in the first four hours after surgery and the trend continued to POD 1, although the difference was not statistically different at POD1, POD2 and POD3 (Fig. 1). Our results are consistent with the 111 ± 62 min half-life of ropivacaine, and the thirty-minute maximum circulating level.20, 21 However there was less opioid consumption in the PMDI group. Similarly Joo et al.22 found no differences in patient pain, satisfaction or range of motion between the PMDI and placebo groups at 12, 24, and 36 h after surgery and on the 2nd and 14th day after surgery, though both were without CFNB.
Although the VAS pain score was not significantly different, less opioid consumption with an equivalent pain score can be interpreted as better pain relief. The most similar trial is a crossover study for staged bilateral TKA in 16 patients.14 The authors used a single shot PMDI and saline CFNB compared to the contralateral receiving saline periarticular injection and CFNB. In contrast to our study, they found no significant difference in patient controlled opioid use or pain in the first 72 h. Their study is limited by the lack of a four-hour time point and a placebo CFNB excluding the possible synergistic effect of the PMDI with CFNB. Interestingly, we actually found significant increases in the mean pain scores in the PMDI group on POD two and three. We postulate that our patients may have had a rebound effect from more vigorous rehabilitation activity allowed by better analgesia during the first 24 h, A similar pattern was seen by Yuenyongviwat et al.,23 where the VAS pain score was higher, but not significantly, in the PA injection treatment group from 48 h to 96 h post-surgery. We found a greater difference in average ROM values between active and active assisted knee mobilization on POD two in the PMDI group (Fig. 3). This reflects more pain in the PDMI group limiting the patient’s ability to actively reach their passive potential. Again this can be attributed to the rebound pain experienced from more vigorous rehab on POD one.
The rebound increase in pain observed in our study on POD2 and POD3 indicates that a more robust pain control protocol is needed during these times, especially as the effect of the PMDI medications wears off. With the trend towards a multimodal protocol that avoids parenteral narcotics, this would mean endorsing a protocol with more preemptive pain medications or changing the pro re nata (PRN) regimen to more of a standing regimen. Currently our patients receive a standing long acting oral narcotics with short acting narcotics and NSAIDs for break through pain. Though this would increase our opioid consumption on POD two and three, it may potentially give the benefit of less pain, more effective physical therapy with better ROM before discharge and shorter length of stay. Another option could be the addition of a liposomal bupivacaine periarticular injection to theoretically decrease pain through POD 2-3, but more studies are needed to validate this assumption in a consistent manner.24, 25, 26Although there were no ‘patient falls’ in our study, adductor canal blocks are another option that can potentially limit the undesirable quadriceps weakness seen in FNB with pain relief by targeting mostly sensory nerves.27, 28
We did not see any significant differences in the side effect profiles or patient satisfaction scores between the two groups. The incidence of opioid-related side effects between groups was the same even during the first 4 h. post-operative when the difference in morphine consumption was significant, possibly due to shorter sample size and low incidence of side effects.
5. Conclusion
The addition of a PMDI injection to our multimodal acute pain treatment regimen for patients undergoing TKA leads to a decrease in opioid consumption within the first four hours after surgery, but does not show a difference in the overall total consumption within the first three days postoperatively. However, the PMDI seems to lead to higher pain scores on the second and third day after surgery possibly attributed to a rebound effect. This observation provides insight and an opportunity for better pain control with other medications during this rebound period. This is important as the patients may start feeling better and confident during the first postoperative day, and any further increase in pain may impact them negatively both mentally and physically. Moreover, this finding can also be a part of preoperative education so that the patients and caregivers are not taken by surprise. The PMDI injection has no significant effect on levels of opioid side-effects or patient satisfaction. This importance of this topic and lack of consensus in TJA literature is evident by the number of comparative randomized controlled trials that continue to emerge regarding the combinations of periarticular PMDI, continuous or single-shot FNB, continuous or single adductor canal blocks, continuous postoperative, intraarticular infusions, and bupivacaine liposomal suspension.29
Conflicts of interest
None.
Contributor Information
Dennis Dimaculangan, Email: dennis.dimaculangan@downstate.edu.
Jin F. Chen, Email: jin.chen@downstate.edu.
Robert B. Borzio, Email: borziorw@gmail.com.
Julio J. Jauregui, Email: juljau@gmail.com.
Vijay J. Rasquinha, Email: vjrasq@hotmail.com.
Aditya V. Maheshwari, Email: Aditya.maheshwari@downstate.edu.
References
- 1.Paul J.E., Arya A., Hurlburt L. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–1162. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 2.Myles P.S., Williams D.L., Hendrata M., Anderson H., Weeks A.M. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84:6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 3.Salinas F.V., Liu S.S., Mulroy M.F. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth Analg. 2006;102:1234–1239. doi: 10.1213/01.ane.0000198675.20279.81. [DOI] [PubMed] [Google Scholar]
- 4.Viscusi E.R., Barrett A.C., Paterson C., Forbes W.P. Efficacy and safety of methylnaltrexone for opioid-induced constipation in patients with chronic noncancer pain: a placebo crossover analysis. Reg Anesth Pain Med. 2015;41(1):93–98. doi: 10.1097/AAP.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fala L., Welz J.A. New perspectives in the treatment of opioid-induced respiratory depression. Am Health Drug Benefits. 2015;8:S51–S63. [PMC free article] [PubMed] [Google Scholar]
- 6.Jannuzzi R.G. Nalbuphine for treatment of opioid-induced pruritus: a systematic review of literature. Clin J Pain. 2016;32:87–93. doi: 10.1097/AJP.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 7.White P.F. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5–S22. doi: 10.1213/01.ANE.0000177099.28914.A7. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila X., Barthelet Y., Biboulet P., Ryckwaert Y., Rubenovitch J., d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Maheshwari A.V., Blum Y.C., Shekhar L., Ranawat A.S., Ranawat C.S. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res. 2009;467:1418–1423. doi: 10.1007/s11999-009-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari A.V., Boutary M., Yun A.G., Sirianni L.E., Dorr L.D. Multimodal analgesia without routine parenteral narcotics for total hip arthroplasty. Clin Orthop Relat Res. 2006;453:231–238. doi: 10.1097/01.blo.0000246545.72445.c4. [DOI] [PubMed] [Google Scholar]
- 11.YaDeau J.T., Cahill J.B., Zawadsky M.W. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg. 2005;101:891–895. doi: 10.1213/01.ANE.0000159150.79908.21. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Singelyn F.J., Ferrant T., Malisse M.F., Joris D. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous femoral nerve sheath block on rehabilitation after unilateral total-hip arthroplasty. Reg Anesth Pain Med. 2005;30:452–457. doi: 10.1016/j.rapm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Macalou D., Trueck S., Meuret P. Postoperative analgesia after total knee replacement: the effect of an obturator nerve block added to the femoral 3-in-1 nerve block. Anesth Analg. 2004;99:251–254. doi: 10.1213/01.ANE.0000121350.09915.84. [DOI] [PubMed] [Google Scholar]
- 14.Ng F.Y., Ng J.K., Chiu K.Y., Yan C.H., Chan C.W. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty: a prospective, crossover, randomized clinical trial. J Arthroplasty. 2012;27:1234–1238. doi: 10.1016/j.arth.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Busch C.A., Shore B.J., Bhandari R. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88:959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 16.Busch C.A., Whitehouse M.R., Shore B.J., MacDonald S.J., McCalden R.W., Bourne R.B. The efficacy of periarticular multimodal drug infiltration in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2152–2159. doi: 10.1007/s11999-009-1198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Cai X.Z., Yan S.G. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2015;30:1281–1286. doi: 10.1016/j.arth.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Kerr D.R., Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008;79:174–183. doi: 10.1080/17453670710014950. [DOI] [PubMed] [Google Scholar]
- 19.Bianconi M., Ferraro L., Traina G.C. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth. 2003;91:830–835. doi: 10.1093/bja/aeg277. [DOI] [PubMed] [Google Scholar]
- 20.Tucker G.T. Pharmacokinetics of local anaesthetics. Br J Anaesth. 1986;58:717–731. doi: 10.1093/bja/58.7.717. [DOI] [PubMed] [Google Scholar]
- 21.Martinsson T., Haegerstrand A., Dalsgaard C.J. Effects of ropivacaine on eicosanoid release from human granulocytes and endothelial cells in vitro. Inflamm Res. 1997;46:398–403. doi: 10.1007/s000110050210. [DOI] [PubMed] [Google Scholar]
- 22.Joo J.H., Park J.W., Kim J.S., Kim Y.H. Is intra-articular multimodal drug injection effective in pain management after total knee arthroplasty? A randomized, double-blinded, prospective study. J Arthroplasty. 2011;26:1095–1099. doi: 10.1016/j.arth.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Yuenyongviwat V., Pornrattanamaneewong C., Chinachoti T., Chareancholvanich K. Periarticular injection with bupivacaine for postoperative pain control in total knee replacement: a prospective randomized double-blind controlled trial. Adv Orthop. 2012;2012:107309. doi: 10.1155/2012/107309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagsby D.T., Ireland P.H., Meneghini R.M. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29:1687–1690. doi: 10.1016/j.arth.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Collis P.N., Hunter A.M., Vaughn M.D., Carreon L.Y., Huang J., Malkani A.L. Periarticular injection after total knee arthroplasty using liposomal Bupivacaine vs a modified Ranawat suspension: a prospective, randomized study. J Arthroplasty. 2016;31:633–636. doi: 10.1016/j.arth.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Schroer W.C., Diesfeld P.G., LeMarr A.R., Morton D.J., Reedy M.E. Does extended-release liposomal bupivacaine better control pain than bupivacaine after total knee arthroplasty (TKA)? A prospective, randomized clinical trial. J Arthroplasty. 2015;30:64–67. doi: 10.1016/j.arth.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Grevstad U., Mathiesen O., Valentiner L.S., Jaeger P., Hilsted K.L., Dahl J.B. Effect of adductor canal block versus femoral nerve block on quadriceps strength, mobilization, and pain after total knee arthroplasty: a randomized, blinded study. Reg Anesth Pain Med. 2015;40:3–10. doi: 10.1097/AAP.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 28.Jaeger P., Zaric D., Fomsgaard J.S. Adductor canal block versus femoral nerve block for analgesia after total knee arthroplasty: a randomized, double-blind study. Reg Anesth Pain Med. 2013;38:526–532. doi: 10.1097/AAP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 29.Moucha C.S., Weiser M.C., Levin E.J. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24:60–73. doi: 10.5435/JAAOS-D-14-00259. [DOI] [PubMed] [Google Scholar]