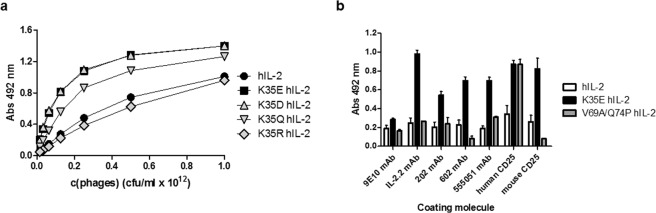
Figure 1.
Effect of mutations at position 35 on IL-2-derived proteins displayed on filamentous phages. Display levels of mutated hIL-2 variants were evaluated by ELISA (a). Equivalent amounts of phage particles (as determined by viral titration) were incubated on polyvinyl chloride microplates coated with the anti-c-myc-tag mAb Myc1-9E10 that recognizes proteins fused to PIII. Bound phages were detected with an anti-M13 mAb conjugated to horseradish peroxidase. Phages displaying non-mutated hIL-2 were used as reference. The quality of the displayed proteins was evaluated in a second ELISA (b). Phages carrying either non-mutated hIL-2, or hIL-2 containing K35E replacement or the double mutation V69A/Q74P, were diluted to reach similar levels of the displayed proteins (according to Myc1-9E10 reactivity in the previous experiment) and incubated on microplates coated with several anti-IL-2 monoclonal antibodies and CD25 (human/mouse). Bound phages were detected as described in (a). Parallel production and ELISA evaluation of phages displaying each variant were independently repeated three times to assess reproducibility of the differences. The evaluation of a representative phage production experiment is shown in each panel. Samples were evaluated by triplicate on ELISA plates.