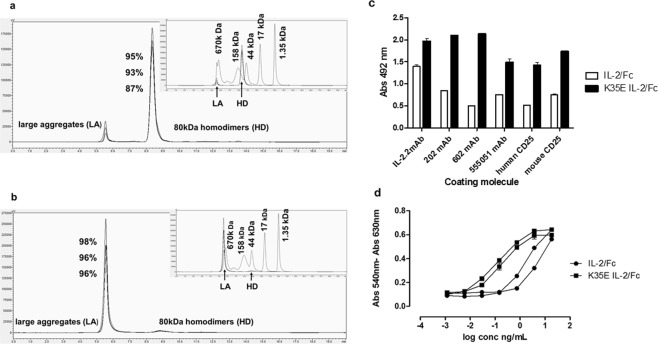
Figure 4.
Differences between IL-2/Fc and K35E IL-2/Fc fusion proteins. The aggregation status of purified fusion proteins was compared by size exclusion chromatography (a,b). The elution profiles of three independent batches of K35E IL-2/Fc (a) and non-mutated IL-2/Fc (b) are shown. A standard comprising five molecules of known molecular weight (thyroglobulin 670 kDa, IgG 158 kDa, ovalbumin 44 kDa, myoglobin 17 kDa, vitamin B12 1.35 kDa) was used for calibration (overlapping between the elution profiles of samples and standards is shown in the inserts). The numbers indicate the relative areas (%) of the main peaks corresponding to homodimers and large aggregates respectively in each kind of sample. The antigenicity and receptor binding properties of IL-2/Fc and K35E IL-2/Fc were compared by ELISA (c) on microplates coated with several anti-IL-2 mAbs and CD25 (human/mouse). Bound proteins were detected with an anti-human Fc antibody conjugated to horseradish peroxidase. The ELISA evaluation of one batch of each fusion protein (both produced in parallel) is shown as a representative example. Samples were evaluated by triplicate in ELISA. The result was confirmed in independent ELISAs with the remaining two batches of each. The ability of two batches of each fusion protein to induce the proliferation of the IL-2-dependent CTLL-2 cell line was evaluated (d), using the Alamar blue dye reduction as an indicator of cell proliferation.