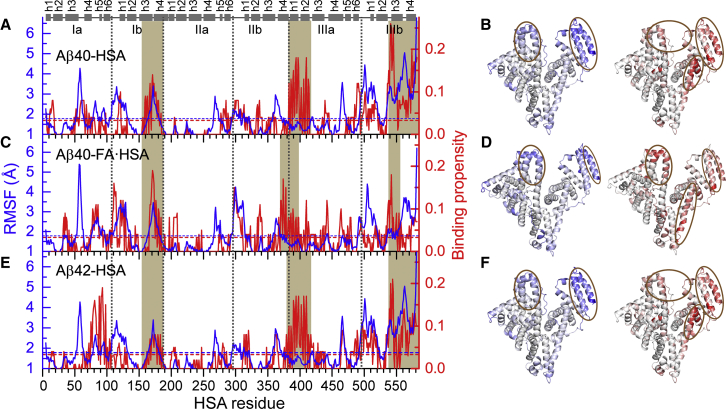
Figure 3.
An apparent correlation between backbone RMSFs of isolated apo or FA·HSA and Aβ binding propensities of HSA residues in three complex systems. (A), (C), and (E) display RMSFs (blue curves) and Aβ binding propensities (red curves) for Aβ40-HSA, Aβ40-FA·HSA, and Aβ42-HSA, respectively. Average values for each system are indicated by blue and red horizontal dashed lines; segments with high RMSFs or high binding propensities are highlighted by brown shading. Subdomain boundaries are indicated by vertical dashed lines; at the top, helices are represented by cylinders. (B), (D), and (F) display the RMSFs (left) and binding propensities (right) as mapped onto the crystal structures of apo or FA·HSA. Regions with high RMSFs and binding propensities are represented by intense blue and red colors, respectively, and highlighted by brown ovals. To see this figure in color, go online.