Abstract
Tendon pathology is a general term used to describe a group of musculoskeletal conditions related to tendons and surrounding structures. There is only limited evidence available regarding the exact aetiology and natural history of tendon pathology. In hypercholesterolaemia environments, lipids could accumulate within the extracellular matrix of the tendon and thus affect the mechanical properties of the tendon. Current evidence suggested that hypercholesterolaemia was an important risk factor in the development and progression of tendon pathology. The severity of hypercholesterolaemia was correlated with the severity of tendon pathology.
The translational potential of this article: Hypercholesterolaemia lead to the structural, inflammatory and mechanical changes in tendons, which predispose hypercholesterolaemia patients to a greater risk of tendon pathology. Measurements of serum cholesterol are suggested to be performed in patients presenting with tendon pathology. The strict control of hypercholesterolaemia would mitigate the development and progression of tendon pathology.
Keywords: Epidemiology, Hypercholesterolaemia, Management, Pathogenesis, Tendon pathology
Background
Tendon pathology is a general term used to describe a group of musculoskeletal conditions related to tendons and surrounding structures [1]. Tendon pathology could be broadly classified into traumatic, degenerative and overuse-related tendinopathy. Rotator cuff tears, Achilles tendinopathy, Achilles tendon rupture, and tennis elbow are common examples of these conditions. Tendon xanthoma is a special type of tendon pathology. There is only limited evidence available regarding the exact aetiology and natural history of tendon pathology [2]. Possible etiologic factors may include aging, overuse, trauma, biomechanical abnormalities, glucocorticoids use, quinolone antibiotics use, microcirculation, and metabolic disorders [3]. Because the pathogenesis of tendon pathology is so complex and involves a variety of biological phenomena, there is no consistently effective treatment available for tendon pathology. Clinically available treatment options may include nonsteroid antiinflammatory drug, corticosteroid injection, platelet-rich plasma injection, low-energy laser stimulation, extracorporeal shock wave therapy, and surgical interventions [3]. Although these approaches may relieve the symptoms in the short term, there is presently limited scientific evidence supporting these therapies and their efficacy.
Hypercholesterolaemia is a systemic metabolic disease characterised by abnormally high levels of cholesterol in the blood. Hypercholesterolaemia is defined as elevated amounts of total cholesterol (≥240 mg/dL) in the blood. Hypercholesterolaemia has well-known impact on vascular systems and internal organs [4]. Recently, the influence of hypercholesterolaemia on musculoskeletal system has attracted much attention. In hypercholesterolaemia environments, lipids could accumulate within the extracellular matrix of the tendon and thus affect the mechanical properties of the tendon [5], [6]. Several studies have explored the relationship between hypercholesterolaemia and tendon pathology. Animal studies indicated that high levels of cholesterol would lead to poorer mechanical properties and adversely affect tendon healing after surgical repair [7], [8], [9], while clinical studies showed inconsistent results on the association between hypercholesterolaemia and tendon pathology [10], [11]. Better understanding of the relationship between hypercholesterolaemia and tendon pathology, the impact of hypercholesterolaemia on tendon structure and healing as well as the mechanisms of hypercholesterolaemia in the development and progression of tendon pathology would aid the development of an effective treatment strategy.
The purpose of this review was (1) to summarise the association between hypercholesterolaemia and tendon pathology, (2) to discuss the pathogenic mechanisms in causing and exacerbating tendon pathology and (3) to explore the potential treatment strategies for tendon pathology in hypercholesterolaemia patients.
Epidemiology of tendon pathology in hypercholesterolaemia patients
The association between hypercholesterolaemia and tendon pathology was reviewed by searching the original research articles in PubMed. The search algorithm was “(tendon or tendinopathy) AND (hyperlipidaemia or dyslipidaemia or hypercholesterolaemia or statin)”. There was significant heterogeneity among the included studies on study design, participants, grouping, sample size and statistical methods. Hypercholesterolaemia is defined as elevated amounts of total cholesterol (≥240 mg/dL) in the blood. The causes of hypercholesterolaemia include diet, lifestyle and genetics [12]. Based on the family history and genetics, hypercholesterolaemia could be divided into familial hypercholesterolaemia (FH) and nonfamilial hypercholesterolaemia (non-FH) [12]. The epidemiology of tendon pathology at FH and non-FH patients was summarised below.
Hypercholesterolaemia and tendon pathology in patients with FH
FH is an inherited genetic disorder, characterised by obviously elevated levels of low-density lipoprotein (LDL) cholesterol, xanthomas and family history of premature atherosclerosis. Based on the family history, FH could be classified as homozygous and heterozygous. The homozygous FH has a prevalence of one per million [13], whereas heterozygous FH affects about one in 200–600 people [14]. Tendon xanthomas are cholesterol deposits within certain tendons, commonly on the Achilles tendons and extensor tendons of the hands [15]. Tendon xanthomas usually appeared in homozygous FH since childhood, while it started to develop after the age of 20 years in patients with heterozygous FH [16]. Approximately 20%–80% of FH patients with genetic diagnosis have tendon xanthomas [17]. It is unknown why some FH patients develop tendon xanthomas and others do not, even with the similar genetic factors. Furthermore, FH could present as Achilles tendinopathy before the development of tendon xanthomas [18]. Measurement of serum cholesterol in patients presenting with painful Achilles tendon could lead to early diagnosis of FH.
Hypercholesterolaemia and tendon pathology in patients without FH
Overuse is considered a major causative factor for tendinopathy. However, a large portion of cases occurred among completely nonactive individuals [19]. People with high body mass index are more likely to suffer from tendinopathy [20]. Though overweight directly affects tendon loading, it is unlikely that increased tendon loading adequately explains these relationships [21]. Alternate mechanisms linking obesity and tendinopathy may be high prevalence of metabolic disorders in the obesity.
Hypercholesterolaemia has been implicated as a risk factor for tendon pathology, but the evidence is mixed. Currently, 15 clinical studies explored the relationship between hyperlipidaemia and tendon pathology. Ten of 15 studies demonstrated that there was an association between dyslipidaemia and tendon pathology (Summarised in Table 1). Gaida et al [22] compared the serum lipid profile between participants with Achilles tendinopathy and those without Achilles tendinopathy and indicated that Achilles tendinopathy was associated with dyslipidaemia and the metabolic syndrome. Abboud et al [23] prospectively collected serum cholesterol and lipid profiles in patients with or without rotator cuff tears and indicated that patients with rotator cuff tears were more likely to have hypercholesterolaemia. Lin et al [10] explored the effect of hyperlipidaemia on the development of rotator cuff disease and demonstrated that hyperlipidaemia was an independent risk factor for rotator cuff disease development. In contrast with these studies, Davis et al [24] compared the serum and synovial fluid lipid profile between participants with intact rotator cuff and rotator cuff tear requiring a repair. The authors indicated that there were no significant differences in any lipid values between patients with and without cuff tears.
Table 1.
The association between hypercholesterolaemia and tendon pathology in patients without familial hypercholesterolaemia.
| First author | Year | Design | Participants | Sample size | Primary findings | Association |
|---|---|---|---|---|---|---|
| Mathiak [57] | 1999 | Case series | Patients with surgical treatment of Achilles tendon ruptures | Total: 41 | Cholesterol levels were found to be elevated in 83% of patients. | Yes |
| Ozgurtas [58] | 2003 | Retrospective cohort study | Study group: with complete ruptures of Achilles tendon Control group: without systemic problems with chronic or acute disease |
Study group: 47 Control group: 26 |
Total cholesterol and low-density lipoprotein cholesterol concentrations of the patients with ATR were higher, and their high-density lipoprotein cholesterol was lower than the control group. | Yes |
| Gaida [22] | 2009 | Prospective cohort study | Study group: with chronic painful midportion Achilles tendinopathy Control group: without a history of tendon injury |
Study group: 60 Control group: 60 |
Higher triglyceride levels, lower % HDL-C, higher TG/HDL-C ratio, and elevated apolipoprotein B concentration. | Yes |
| Abboud [23] | 2010 | Prospective cohort study | Study group: with rotator cuff tears Control group: with shoulder pain but without tears |
Study group: 74 Control group: 73 |
TC, TG, and LDL-C concentrations of the patients with rotator cuff tendon tears were significantly higher than the control group. The high-density lipoprotein cholesterol showed a trend to being lower than the control group. | Yes |
| Longo [11] | 2010 | Case–control study | Study group: arthroscopic repair of a rotator cuff tear Control group: arthroscopic meniscectomy for a meniscal tear |
Study group: 120 Control group: 120 |
There was no statistically significant difference in serum TG and TC concentration. | No |
| Rechardt [59] | 2013 | Cross-sectional study | Patients with incipient upper extremity pain with symptom duration of less than 1 month | Total: 163 | Obesity, high-density lipoprotein cholesterol and triglycerides were associated with pain intensity. | Yes |
| Abate [60] | 2014 | Cross-sectional study | Group 1: female patient with lower limb diseases older than 44 years and with regular menstrual cycles Group 2: postmenopause 2–7 years |
Group 1: 110 Group 2: 122 |
High TG and low HDL-C were associated with an increased risk of asymptomatic rotator cuff tears. This was not statistically significant with TC. | Yes |
| Oliva [61] | 2014 | Retrospective observational study | Patients with nontraumatic rotator cuff tear | Total: 441 | High proportions of patients with nontraumatic rotator cuff tears had hypercholesterolaemia. High portions of patients with hypercholesterolaemia took cholesterol-lowering medications. | Yes |
| Djerbi [62] | 2015 | Prospective cohort study | Study group: patients undergoing arthroscopic rotator cuff repair Control group: operated on other parts but not shoulder |
Study group: 206 Control group: 100 |
Patients with dyslipidaemia had significantly higher odds ratio of rotator cuff tears. | Yes |
| Lin [10] | 2015 | Retrospective cohort study | Randomly selected from national health research database | Total: 498678 | Hyperlipidaemia was an independent risk factor for rotator cuff disease development. An increased risk also existed in patients with hyperlipidaemia with/without statin use. Statin use was associated with a lower risk of developing rotator cuff diseases when compared with no statin use. | Yes |
| Davis [24] | 2016 | Prospective cohort study | Study group: rotator cuff tear requiring a repair Control group: with intact rotator cuff |
Study group: 40 Control group: 37 |
There were no significant differences in any lipid values between patients with rotator cuff and those without a tear. | No |
| Kim [63] | 2016 | Retrospective cohort study, | Study group: supraspinatus tendinopathy with dyslipidaemia Control group: supraspinatus tendinopathy without dyslipidaemia | Study group: 49 Control group: 50 |
Rotator cuff tears were more frequent in the hyperlipidaemia group although statistical analysis showed no significant difference. Patients with hyperlipidaemia had significantly less improvement in pain level. | Yes |
| Abate [64] | 2017 | Case series | Group 1: with monolateral rotator cuff tear Group 2: with bilateral rotator cuff tear |
Group 1: 111 Group 2: 69 |
There was no association of bilateral rotator cuff tears with hypercholesterolaemia and statin therapy. | No |
| Applegate [65] | 2017 | Cross-sectional study | Workers were recruited from 17 diverse production facilities | Total: 1226 | Hypercholesterolaemia was statistically associated with glenohumeral joint pain, but not rotator cuff tendinopathy. | No |
| Juge [66] | 2017 | Retrospective cohort study | Group 1: with rotator cuff–related osteoarthritis Group 2: with primary shoulder osteoarthritis |
Group 1: 48 Group 2: 99 |
There were no significant difference in the rate of dyslipidaemia between rotator cuff–related osteoarthritis and primary shoulder osteoarthritis. | No |
LDL-C = low-density lipoprotein cholesterol; ATR = Achilles tendon ruptures; HDL-C = high-density lipoprotein cholesterol; TG = triglyceride; TC = Total cholesterol.
For FH patients, hypercholesterolaemia is associated with xanthoma formation. While for non-FH patients, there is a potential role of hypercholesterolaemia in predisposing to tendon pathology in the general population. It looks like that people with worse hypercholesterolaemia (as FH) are more likely to suffer from tendon pathology, even tendon xanthomas.
Pathogenic mechanisms
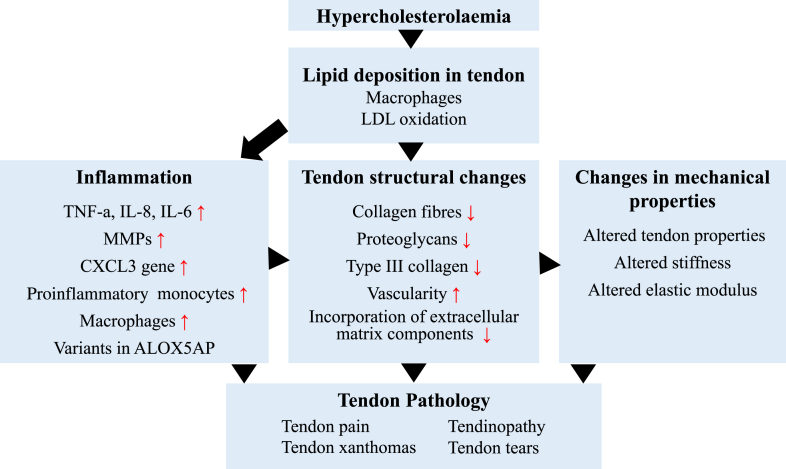
Extracellular matrix remodelling and inflammation are reported to be two key factors in the development of atherosclerosis in hypercholesterolaemia patients [25]. There is limited evidence regarding the potential pathogenic mechanisms of hypercholesterolaemia on tendon. It is necessary to summarise the current evidence of the mechanisms of lipid deposition in tendon. Furthermore, the pathogenic mechanisms of hypercholesterolaemia on tendon were summarised from the following three points: inflammation, tendon structural changes and changes in mechanical properties (Figure 1).
Figure 1.
The pathogenic mechanisms of hypercholesterolaemia on tendon.
MMPs = matrix metalloproteinases; LDL = low-density lipoprotein; TNF-a = tumour necrosis factor alpha; IL-8 = interleukin 8; IL-6 = interleukin 6.
The mechanisms of lipid deposition in tendon
The direct impact of hypercholesterolaemia on tendon is cholesterol deposits within tendon tissues, along with the changes of tendon mechanical properties [7], [15]. Tendon xanthomas are usually accompanied by an increase in tendon size, correlated with the degree of hypercholesterolaemia [26]. The main constituents of tendon xanthomas are lipids and collagen. In tendon xanthomas, Kruth [15] indicated that unesterified cholesterol accumulated predominantly extracellularly in the tendon as human atherosclerotic lesions, while esterified cholesterol and triglyceride accumulated both extracellularly and intracellularly. Lipid analysis of tendon xanthomas indicated that the lipid was composed of 55% free cholesterol, 28% cholesterol esters and 13% phospholipids [27], [28]. Bhattacharyya et al [29] explored the turnover of xanthoma cholesterol in hypercholesterolaemia patients and suggested total exchangeability of cholesterol between plasma and xanthomas. Accordingly, lipids in tendon xanthomas are more likely to be derived from the circulation rather than from local synthesis, secretion or cell death. These findings were supported by the study of Armstrong et al [30] indicating active uptake of LDL by the lesions within xanthomas. Sugiyama et al [31] determined the presence and distribution of lipoproteins by immunohistochemical methods and indicated that oxidatively modified low-density lipoprotein (oxLDL) appeared to have a similar distribution in xanthoma to that of macrophages. Furthermore, the study demonstrated that oxLDL was associated with macrophages and occurred intracellularly. LDL was detected extracellularly, with a distribution that was different from that of oxLDL [31]. It was assumed that LDL derived from plasma was trapped in the tendon matrix and oxidised by macrophages or other cells. The majority of the xanthoma cells were considered to be derived from macrophages after taking up oxLDL.
Inflammation in the pathological process of tendon pathology
The role of inflammation in the development and progression of cardiovascular diseases is well established. Thus, inflammation is suggested to be the main pathological mechanism of tendon pathology in hypercholesterolaemia patients. Artieda et al [32] indicated that macrophages derived from patients with tendon xanthomas were more likely to form foam cells than macrophages from patients without tendon xanthomas. FH patients with tendon xanthomas showed increased serum tryptase, tumour necrosis factor-α, interleukin-8, and interleukin-6 concentrations than FH patients without tendon xanthomas. These authors proposed that tendon xanthomas formation was associated with higher intracellular lipid content and higher inflammatory response of macrophages. In addition, Oosterveer et al [33] indicated that variants in the ALOX5AP (5-lipoxygenase activating protein) gene were associated with the presence of tendon xanthomas in FH patients. ALOX5AP is involved in the biosynthesis of leukotrienes by mediating the activity of 5-lipoxygenase. Leukotrienes promote leucocyte chemotaxis and increase vascular permeability. The authors concluded that inflammation was a pathogenic factor of tendon xanthomas. CXCL3 is a chemokine belonging to the growth-regulated oncogene family, which acts as mediators in allergy, inflammation and immunity. The study by Martin-Fuentes et al [34] indicated that chemokines belonging to the CXC family could play an important role in the aetiology of tendon xanthomas. CXCL3 was a possible biological marker of onset and development of tendon xanthomas. Hjuler Nielsen et al [35] concluded that lipoprotein-associated oxidative stress was involved in tendon xanthomas by inducing proinflammatory monocytes and increased release of MMPs. These above studies consistently showed that inflammation was involved in the development of tendon xanthomas.
Tendon structural changes in hypercholesterolaemia environment
Hypercholesterolaemia environment would alter tendon homoeostasis. Hypercholesterolaemia was associated with decreased synthesis of noncollagenous proteins and decreased incorporation of extracellular matrix components [36]. Nakano et al [37] explored the pathogenesis of tendon xanthomas in rabbits and showed that a large number of blood vessels were seen in the xanthomas tissues. Immunohistochemical evaluation revealed that the xanthoma plaques contained endothelial cells and macrophages. Nunes et al [38] investigated the effects of hypercholesterolaemia on the collagen composition of urinary bladder wall and indicated that hypercholesterolaemia induced morphological alterations of collagen fibres and the amounts of type III collagen. Oberkersch et al showed that hypercholesterolaemia affected proteoglycans synthesis [39]. In summary, current evidence suggested that hypercholesterolaemia could alter the tendon microenvironment via local changes in protein synthesis and extracellular matrix remodelling.
Changes in mechanical properties of tendon in hypercholesterolaemia environment
Hypercholesterolaemia could affect the mechanical strength of plaques by inducing local collagen loss and render atherosclerotic plaques prone to rupture [40]. The effects of hypercholesterolaemia on biomechanical properties of tendon have been explored in animal models. Hypercholesterolaemia is believed to contribute to increased tendon injury in several ways. Beason et al [7] explored the effect of high cholesterol on tendon properties in mice and indicated that there was a detrimental effect of hypercholesterolaemia on tendon properties. In addition, another study by Beason et al [8] explored the effect of hypercholesterolaemia on supraspinatus tendon elastic mechanical properties in mice, rats and monkeys. The authors concluded that hypercholesterolaemia could lead to an increase in stiffness and elastic modulus of the supraspinatus tendons in these species. Hypercholesterolaemia-related changes on mechanical properties might lead to the increased rates of tendon injury. Chung et al [41] explored the effect of hyperlipidaemia on fatty infiltration and tendon-to-bone healing in a rabbit model and demonstrated that hyperlipidaemia had a deleterious effect on fatty infiltration and tendon-to-bone healing. Hypercholesterolaemia could alter the biomechanical properties of tendon and thus render tendon prone to injury.
Managements for tendon pathology in hypercholesterolaemia patients
The best approach towards tendon pathology in patients with hypercholesterolaemia is treating the metabolic disorder of lipid metabolism. When lifestyle changes are not effective in lowering the serum cholesterol, statins are recommended for the first-line treatment. Statins are the most widely prescribed medications to treat hyperlipidaemia and reduce the risk of cardiovascular diseases and related mortality [42], [43]. Several studies have demonstrated that statins are effective in decreasing the size of tendon xanthomas [44], [45]. Statins work by lowering serum cholesterol and are therefore associated with mobilisation of cholesterol from tendon xanthomas. In the patients without FH, use of statins is associated with a lower risk of developing tendon pathology when compared with no statin use [10]. However, statins are known to have a potentially deleterious effect on muscle. Use of statins was associated with myalgia, muscle injury, increase in creatine kinase and even rarer rhabdomyolysis [46]. In addition, several studies suggested that use of statins might be associated with tendon pathology and tendon ruptures [47], [48]. Thus, the benefits of statins on tendon pathology needed to be balanced with the potential adverse effects on tendon.
The goal of cholesterol reduction in patients with FH is minimal reduction ≥50% of LDL cholesterol or at ideal LDL cholesterol level [14]. When high-dose statins are not effective in lowering the serum cholesterol, second-line drugs such as ezetimibe, PCSK9 inhibitors, niacin or bile acid sequestrants should be added to further decrease the cholesterol levels [14]. The combination of statins and ezetimibe decreases both the production of cholesterol in the liver and absorption of dietary cholesterol in small intestine [49]. Adding PCSK9 inhibitor should be considered if the ideal goal of cholesterol reduction could not be reached after treatment with high-dose statins plus ezetimibe [50]. Bile acid sequestrants and niacin are optional depending on the availability, toxic effects, and costs. Other approaches including lomitapide, mipomersen, lipoprotein aphaeresis and liver transplantation are not commonly prescribed and could be considered when the above triple-drug therapies (statins, ezetimibe and PCSK9 inhibitor) are not effective [14]. Finally, surgical resection of the tendon xanthomas might be considered in some severe cases [51].
Besides control of hypercholesterolaemia, several approaches were suggested to promote tendon regeneration after removing primary diseases. Popular injectable substances included growth factors, platelet-rich plasma, autologous blood, mesenchymal stem cells, stromal vascular fraction and bone marrow aspirate concentrate. A meta-analysis of controlled studies has shown that platelet-rich plasma is a safe and promising therapy in the treatment of recalcitrant patellar tendinopathy [52]. Another study indicated that platelet-rich plasma could ameliorate the pain of tendinopathy in the intermediate–long term compared with the control interventions [53]. A recent pilot study revealed that there was a therapeutic value of mesenchymal stem cell injection for treating chronic tendinopathy [54]. Usuelli et al [55] indicated that intratendinous adipose-derived stromal vascular fraction injection provided a safe and efficacious treatment for Achilles tendinopathy. A recent study systematically reviewed the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology [56]. The authors concluded that there were only limited clinical studies available and future randomised controlled studies were highly needed.
Conclusion
Current evidence generally suggested that hypercholesterolaemia was an important risk factor in the development and progression of tendon pathology. The severity of hypercholesterolaemia was correlated with the severity of tendon pathology. Hypercholesterolaemia lead to the structural, inflammatory and mechanical changes in tendons, which predispose hypercholesterolaemia patients to a greater risk of tendon pathology. The strict control of hypercholesterolaemia would mitigate the development and progression of tendon pathology.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Funding/Acknowledgement
This study was funded by the National Natural Science Foundation of China (81500358 and 81501898).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.07.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Abate M., Silbernagel K.G., Siljeholm C., Di Iorio A., De Amicis D., Salini V. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaux J.F., Forthomme B., Goff C.L., Crielaard J.M., Croisier J.L. Current opinions on tendinopathy. J Sports Sci Med. 2011;10:238–253. [PMC free article] [PubMed] [Google Scholar]
- 3.Li H.Y., Hua Y.H. Achilles tendinopathy: current concepts about the basic science and clinical treatments. Biomed Res Int. 2016;2016:6492597. doi: 10.1155/2016/6492597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan S., Zhang Y., Wu S.J., Jiang L.Z., Zhang J., Gan Y. Atorvastatin attenuates inflammatory infiltration and vascular remodeling in lung of hypercholesterolemia rabbits. Exp Lung Res. 2010;36:573–592. doi: 10.3109/01902141003739715. [DOI] [PubMed] [Google Scholar]
- 5.Soslowsky L.J., Fryhofer G.W. Tendon homeostasis in hypercholesterolemia. Adv Exp Med Biol. 2016;920:151–165. doi: 10.1007/978-3-319-33943-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Taylor B., Cheema A., Soslowsky L. Tendon pathology in hypercholesterolemia and familial hypercholesterolemia. Curr Rheumatol Rep. 2017;19:76. doi: 10.1007/s11926-017-0704-2. [DOI] [PubMed] [Google Scholar]
- 7.Beason D.P., Abboud J.A., Kuntz A.F., Bassora R., Soslowsky L.J. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29:380–383. doi: 10.1002/jor.21255. [DOI] [PubMed] [Google Scholar]
- 8.Beason D.P., Hsu J.E., Marshall S.M., McDaniel A.L., Temel R.E., Abboud J.A. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg. 2013;22:681–686. doi: 10.1016/j.jse.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beason D.P., Tucker J.J., Lee C.S., Edelstein L., Abboud J.A., Soslowsky L.J. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg. 2014;23:867–872. doi: 10.1016/j.jse.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T.T., Lin C.H., Chang C.L., Chi C.H., Chang S.T., Sheu W.H. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015;43:2126–2132. doi: 10.1177/0363546515588173. [DOI] [PubMed] [Google Scholar]
- 11.Longo U.G., Franceschi F., Spiezia F., Forriol F., Maffulli N., Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med. 2010;44:948–951. doi: 10.1136/bjsm.2008.056440. [DOI] [PubMed] [Google Scholar]
- 12.Xiang R., Fan L.L., Lin M.J., Li J.J., Shi X.Y., Jin J.Y. The genetic spectrum of familial hypercholesterolemia in the central south region of China. Atherosclerosis. 2017;258:84–88. doi: 10.1016/j.atherosclerosis.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Raal F.J., Santos R.D. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–268. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Santos R.D., Gidding S.S., Hegele R.A., Cuchel M.A., Barter P.J., Watts G.F. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–861. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 15.Kruth H.S. Lipid deposition in human tendon xanthoma. Am J Pathol. 1985;121:311–315. [PMC free article] [PubMed] [Google Scholar]
- 16.Pejic R.N. Familial hypercholesterolemia. Ochsner J. 2014;14:669–672. [PMC free article] [PubMed] [Google Scholar]
- 17.Oosterveer D.M., Versmissen J., Yazdanpanah M., Hamza T.H., Sijbrands E.J. Differences in characteristics and risk of cardiovascular disease in familial hypercholesterolemia patients with and without tendon xanthomas: a systematic review and meta-analysis. Atherosclerosis. 2009;207:311–317. doi: 10.1016/j.atherosclerosis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Beeharry D., Coupe B., Benbow E.W., Morgan J., Kwok S., Charlton-Menys V. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis. 2006;65:312–315. doi: 10.1136/ard.2005.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolf C., Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18:565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi F., Papalia R., Paciotti M., Franceschetti E., Di Martino A., Maffulli N. Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol. 2014;2014:670262. doi: 10.1155/2014/670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaida J.E., Cook J.L., Bass S.L. Adiposity and tendinopathy. Disabil Rehabil. 2008;30:1555–1562. doi: 10.1080/09638280701786864. [DOI] [PubMed] [Google Scholar]
- 22.Gaida J.E., Alfredson L., Kiss Z.S., Wilson A.M., Alfredson H., Cook J.L. Dyslipidemia in Achilles tendinopathy is characteristic of insulin resistance. Med Sci Sports Exerc. 2009;41:1194–1197. doi: 10.1249/MSS.0b013e31819794c3. [DOI] [PubMed] [Google Scholar]
- 23.Abboud J.A., Kim J.S. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468:1493–1497. doi: 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis D.E., Narzikul A., Sholder D., Lazarus M., Namdari S., Abboud J. Shoulder synovial fluid lipoprotein levels and their relationship to the rotator cuff. Med Sci Sports Exerc. 2017;49:396–402. doi: 10.1249/MSS.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 25.Schwanekamp J.A., Lorts A., Vagnozzi R.J., Vanhoutte D., Molkentin J.D. Deletion of periostin protects against atherosclerosis in mice by altering inflammation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2016;36:60–68. doi: 10.1161/ATVBAHA.115.306397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsouli S.G., Kiortsis D.N., Argyropoulou M.I., Mikhailidis D.P., Elisaf M.S. Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest. 2005;35:236–244. doi: 10.1111/j.1365-2362.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- 27.Tall A.R., Small D.M., Lees R.S. Interaction of collagen with the lipids of tendon xanthomata. J Clin Invest. 1978;62:836–846. doi: 10.1172/JCI109196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermeer B.J., Mateysen A.A., van Gent C.M., van Sabben R.M., Emeis J.J. The lipid composition and localization of free and esterified cholesterol in different types of xanthomas. J Invest Dermatol. 1982;78:305–308. doi: 10.1111/1523-1747.ep12507376. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya A.K., Connor W.E., Mausolf F.A., Flatt A.D. Turnover of xanthoma cholesterol in hyperlipoproteinemia patients. J Lab Clin Med. 1976;87:503–518. [PubMed] [Google Scholar]
- 30.Armstrong M.L., Mathur S.N., Sando G.N., Megan M.B. Lipid metabolism in xanthomatous skin of hypercholesterolemic rabbits. Am J Pathol. 1986;125:339–348. [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama N., Marcovina S., Gown A.M., Seftel H., Joffe B., Chait A. Immunohistochemical distribution of lipoprotein epitopes in xanthomata from patients with familial hypercholesterolemia. Am J Pathol. 1992;141:99–106. [PMC free article] [PubMed] [Google Scholar]
- 32.Artieda M., Cenarro A., Junquera C., Lasierra P., Martinez-Lorenzo M.J., Pocovi M. Tendon xanthomas in familial hypercholesterolemia are associated with a differential inflammatory response of macrophages to oxidized LDL. FEBS Lett. 2005;579:4503–4512. doi: 10.1016/j.febslet.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 33.Oosterveer D.M., Versmissen J., Yazdanpanah M., van der Net J.B., Defesche J.C., Kastelein J.J. 5-Lipoxygenase activating protein (ALOX5AP) gene variants associate with the presence of xanthomas in familial hypercholesterolemia. Atherosclerosis. 2009;206:223–227. doi: 10.1016/j.atherosclerosis.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Fuentes P., Civeira F., Solanas-Barca M., Garcia-Otin A.L., Jarauta E., Cenarro A. Overexpression of the CXCL3 gene in response to oxidized low-density lipoprotein is associated with the presence of tendon xanthomas in familial hypercholesterolemia. Biochem Cell Biol. 2009;87:493–498. doi: 10.1139/o09-006. [DOI] [PubMed] [Google Scholar]
- 35.Hjuler Nielsen M., Irvine H., Vedel S., Raungaard B., Beck-Nielsen H., Handberg A. Elevated atherosclerosis-related gene expression, monocyte activation and microparticle-release are related to increased lipoprotein-associated oxidative stress in familial hypercholesterolemia. PLoS One. 2015;10:e0121516. doi: 10.1371/journal.pone.0121516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronnemaa T., Juva K., Kulonen E. Effect of hyperlipidemic rat serum on the synthesis of collagen by chick embryo fibroblasts. Atherosclerosis. 1975;21:315–324. doi: 10.1016/0021-9150(75)90045-3. [DOI] [PubMed] [Google Scholar]
- 37.Nakano A., Kinoshita M., Okuda R., Yasuda T., Abe M., Shiomi M. Pathogenesis of tendinous xanthoma: histopathological study of the extremities of Watanabe heritable hyperlipidemic rabbits. J Orthop Sci. 2006;11:75–80. doi: 10.1007/s00776-005-0976-7. [DOI] [PubMed] [Google Scholar]
- 38.Nunes R.L., Bruschini H., Utsunomia K., Silveira M.A., Teodoro W.R., Leite K.R. Influence of a hypercholesterolemic diet on the collagen composition of the bladder wall extracellular matrix in rats. Histol Histopathol. 2012;27:745–752. doi: 10.14670/HH-27.745. [DOI] [PubMed] [Google Scholar]
- 39.Oberkersch R., Maccari F., Bravo A.I., Volpi N., Gazzaniga S., Calabrese G.C. Atheroprotective remodelling of vascular dermatan sulphate proteoglycans in response to hypercholesterolaemia in a rat model. Int J Exp Pathol. 2014;95:181–190. doi: 10.1111/iep.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rekhter M.D., Hicks G.W., Brammer D.W., Hallak H., Kindt E., Chen J. Hypercholesterolemia causes mechanical weakening of rabbit atheroma : local collagen loss as a prerequisite of plaque rupture. Circ Res. 2000;86:101–108. doi: 10.1161/01.res.86.1.101. [DOI] [PubMed] [Google Scholar]
- 41.Chung S.W., Park H., Kwon J., Choe G.Y., Kim S.H., Oh J.H. Effect of hypercholesterolemia on fatty infiltration and quality of tendon-to-bone healing in a rabbit model of a chronic rotator cuff tear: electrophysiological, biomechanical, and histological analyses. Am J Sports Med. 2016;44:1153–1164. doi: 10.1177/0363546515627816. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W., Zheng X.L., Jiang Z.N., Liao X.B., Zhao S.P. Risk factors associated with atherogenic dyslipidemia in the presence of optimal statin therapy. Int J Cardiol. 2017;248:355–360. doi: 10.1016/j.ijcard.2017.06.105. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S., Peng D. Efficacy and safety of rosuvastatin versus atorvastatin in high-risk Chinese patients with hypercholesterolemia: a randomized, double-blind, active-controlled study. Curr Med Res Opin. 2018;34:227–235. doi: 10.1080/03007995.2017.1371584. [DOI] [PubMed] [Google Scholar]
- 44.Illingworth D.R., Cope R., Bacon S.P. Regression of tendon xanthomas in patients with familial hypercholesterolemia treated with lovastatin. South Med J. 1990;83:1053–1057. doi: 10.1097/00007611-199009000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Heath K.E., Gudnason V., Humphries S.E., Seed M. The type of mutation in the low density lipoprotein receptor gene influences the cholesterol-lowering response of the HMG-CoA reductase inhibitor simvastatin in patients with heterozygous familial hypercholesterolaemia. Atherosclerosis. 1999;143:41–54. doi: 10.1016/s0021-9150(98)00274-3. [DOI] [PubMed] [Google Scholar]
- 46.Ballard K.D., Taylor B.A., Thompson P.D. Statin-associated muscle injury. Eur J Prev Cardiol. 2015;22:1161. doi: 10.1177/2047487315586096. [DOI] [PubMed] [Google Scholar]
- 47.Teichtahl A.J., Brady S.R., Urquhart D.M., Wluka A.E., Wang Y., Shaw J.E. Statins and tendinopathy: a systematic review. Med J Aust. 2016;204:115–121 e1. doi: 10.5694/mja15.00806. [DOI] [PubMed] [Google Scholar]
- 48.Deren M.E., Klinge S.A., Mukand N.H., Mukand J.A. Tendinopathy and tendon rupture associated with statins. JBJS Rev. 2016;4 doi: 10.2106/JBJS.RVW.15.00072. [DOI] [PubMed] [Google Scholar]
- 49.Lin M., Dai H., Zhao S. Long-term atorvastatin-ezetimibe-probucol triple therapy for homozygous familial hypercholesterolaemia from early childhood. Cardiol Young. 2016;26:197–201. doi: 10.1017/S1047951115000591. [DOI] [PubMed] [Google Scholar]
- 50.Shen L., Peng H., Xu D., Zhao S. The next generation of novel low-density lipoprotein cholesterol-lowering agents: proprotein convertase subtilisin/kexin 9 inhibitors. Pharmacol Res. 2013;73:27–34. doi: 10.1016/j.phrs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Moroney P.J., Besse J.L. Resection of bilateral massive Achilles tendon xanthomata with reconstruction using a flexor hallucis longus tendon transfer and Bosworth turndown flap: a case report and literature review. Foot Ankle Surg. 2012;18:e25–e28. doi: 10.1016/j.fas.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Liddle A.D., Rodriguez-Merchan E.C. Platelet-rich plasma in the treatment of patellar tendinopathy: a systematic review. Am J Sports Med. 2015;43:2583–2590. doi: 10.1177/0363546514560726. [DOI] [PubMed] [Google Scholar]
- 53.Andia I., Latorre P.M., Gomez M.C., Burgos-Alonso N., Abate M., Maffulli N. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99–115. doi: 10.1093/bmb/ldu007. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.Y., Kim W., Lim C., Chung S.G. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33:2995–3005. doi: 10.1002/stem.2110. [DOI] [PubMed] [Google Scholar]
- 55.Usuelli F.G., Grassi M., Maccario C., Vigano M., Lanfranchi L., Alfieri Montrasio U. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc. 2018 Jul;26(7):2000–2010. doi: 10.1007/s00167-017-4479-9. [DOI] [PubMed] [Google Scholar]
- 56.Imam M.A., Holton J., Horriat S., Negida A.S., Grubhofer F., Gupta R. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J. 2017;3:58. doi: 10.1051/sicotj/2017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathiak G., Wening J.V., Mathiak M., Neville L.F., Jungbluth K. Serum cholesterol is elevated in patients with Achilles tendon ruptures. Arch Orthop Trauma Surg. 1999;119:280–284. doi: 10.1007/s004020050410. [DOI] [PubMed] [Google Scholar]
- 58.Ozgurtas T., Yildiz C., Serdar M., Atesalp S., Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta. 2003;331:25–28. doi: 10.1016/s0009-8981(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 59.Rechardt M., Shiri R., Lindholm H., Karppinen J., Viikari-Juntura E. Associations of metabolic factors and adipokines with pain in incipient upper extremity soft tissue disorders: a cross-sectional study. BMJ Open. 2013;3:e003036. doi: 10.1136/bmjopen-2013-003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abate M., Schiavone C., Di Carlo L., Salini V. Prevalence of and risk factors for asymptomatic rotator cuff tears in postmenopausal women. Menopause. 2014;21:275–280. doi: 10.1097/GME.0b013e31829638e3. [DOI] [PubMed] [Google Scholar]
- 61.Oliva F., Osti L., Padulo J., Maffulli N. Epidemiology of the rotator cuff tears: a new incidence related to thyroid disease. Muscles Ligaments Tendons J. 2014;4:309–314. [PMC free article] [PubMed] [Google Scholar]
- 62.Djerbi I., Chammas M., Mirous M.P., Lazerges C., Coulet B. Impact of cardiovascular risk factor on the prevalence and severity of symptomatic full-thickness rotator cuff tears. Orthop Traumatol Surg Res. 2015;101:S269–S273. doi: 10.1016/j.otsr.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Kim J.M., Kim M.W., Do H.J. Influence of hyperlipidemia on the treatment of supraspinatus tendinopathy with or without tear. Ann Rehabil Med. 2016;40:463–469. doi: 10.5535/arm.2016.40.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abate M., Di Carlo L., Salini V., Schiavone C. Risk factors associated to bilateral rotator cuff tears. Orthop Traumatol Surg Res. 2017;103:841–845. doi: 10.1016/j.otsr.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 65.Applegate K.A., Thiese M.S., Merryweather A.S., Kapellusch J., Drury D.L., Wood E. Association between cardiovascular disease risk factors and rotator cuff tendinopathy: a cross-sectional study. J Occup Environ Med. 2017;59:154–160. doi: 10.1097/JOM.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 66.Juge P.A., Berard L., Kotti S., Doursounian L., Sautet A., Simon T. Cardiometabolic risk factors in primary centred and rotator cuff-related shoulder osteoarthritis: a comparative study. RMD Open. 2017;3:e000429. doi: 10.1136/rmdopen-2016-000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.