Abstract
Objective
Glucocorticoids (GCs) are commonly prescribed as treatment for chronic inflammatory diseases. Prolonged use of GCs is a common cause of atraumatic osteonecrosis (ON) and secondary osteoporosis. Currently, there is no effective treatment for this disease; therefore, a reliable animal model would be useful to study both the pathology and novel treatment strategies for patients with the disease. The aim of this study was to establish a validated, reproducible model of GC-induced ON and bone loss in two different mouse strains (BALB/c and C57BL/6).
Methods
Seven-week-old male BALB/c (n = 32) and male C57BL/6 mice (n = 32) were randomised into placebo or GC groups and treated with daily 4 mg/L oral dexamethasone in drinking water for 90 days. Study outcome measures included histologic assessment of ON of the distal femur, bone mass and mechanical strength of tibia and lumbar vertebral body, osteoclast number, biochemical measure of bone formation and bone marrow fat quantitation.
Results
GC-induced ON lesions were observed in the distal femur in 47% of the male BALB/c mice and 25% of the male C57BL/6 mice. GC treatment decreased the trabecular bone volume and serum pro-collagen type 1N-protease (P1NP) in BALB/c mice compared with the placebo (p < 0.05) and reduced tibial bone strength in both BALB/c and C57BL/6 mice. GC-treated BALB/c mice had significantly greater marrow fat levels compared to the placebo group.
Conclusion
GC-induced ON was more prevalent in the male BALB/c mice compared to the male C57BL/6 mice. GC treatment significantly reduced bone mass, bone formation measured by P1NP, bone strength and increased marrow fat levels in male BALB/c mice. Therefore, the use of male BALB/c mice strain is recommended for both diagnostic and therapeutic studies for the prevention and treatment of ON and bone loss following prolonged treatment with GCs.
The Translational Potential of this Article
GCs are commonly used to treat patients with various chronic inflammatory diseases, and this is associated with both the development of ON and bone loss. Our study confirmed that the BALB/c mouse strain treated for 90 days with GC may be useful for developing novel treatments for ON.
Keywords: Animal model, Bone loss, Glucocorticoids, Osteonecrosis
Introduction
Glucocorticoids (GCs) are commonly used for the treatment of various chronic inflammatory diseases including rheumatoid arthritis, systemic lupus erythematosus and systemic vasculitis [1]. However, long-term GC use is the common cause of GC-induced avascular osteonecrosis (ON) and GC-induced secondary osteoporosis [2]. It has been reported that between 5 and 40% of patients treated with long-term GC develop ON [3], and between 30 and 50% of patients develop GC-induced secondary osteoporosis [4]. In addition, GC-induced ON of the femoral head increases the risk of fractures and may lead to femoral head collapse, which often requires surgical treatments including total hip arthroplasty, bone grafting and osteotomy [2], [5], [6], [7]. Therefore, preclinical models of GC-induced ON that resemble the human pathology are useful for the development of novel and effective treatment modalities for patients with the disease.
There have been considerable studies on the likely mechanisms of GC-induced ON, including the increased number of enlarged adipose cells and fat emboli [8], intravascular thrombi formation [9], reduced microcirculation [10], osteocyte apoptosis [11] and GC suppression of genes involved in perilacunar remodelling [12]. Other studies suggest that GC-induced ON impairs bone marrow blood flow by increased intravascular coagulation and extravascular fat deposition [13]. Previous studies have reported on the adverse effects of GCs on bone quality including reduced bone density [2], bone mass [4] and bone strength [14]. Furthermore, it has been shown that GC effects on bone quality and bone strength may be attributed to reduced bone vascularity, blood flow and the amount of water within bone [15].
Previously described GC-induced animal models of ON have reported inconsistent results on the incidence of ON [16], [17] and GC effects on bone [18], [19]. Therefore, the objective of this study was to establish a validated, reproducible animal model to assess both GC-induced ON and the development of bone loss in two different strains of male mice.
Materials and methods
Animals
Seven-week-old male BALB/c (n = 32) and C57BL/6 (n = 32) mice were randomised into either the GC group or the placebo group. We treated both mouse strains with oral dexamethasone (4 mg/L, Sigma–Aldrich, St. Louis, MO) in drinking water for 90 days. The GC-treated BALB/c and C57BL/6 mice received stock solutions of 5.3 mL of 79 mg sulfamethoxazole and 16 mg trimethoprim in 400 mL drinking water per body kilogramme every 24 hours from Day 45 to prevent infections due to prolonged GC treatment. The placebo mice received fresh drinking water. At the end of the study, mice were euthanised using CO2.
Ethics statement: All the animals were treated in accordance with United States Department of Agriculture (USDA) animal care guidelines with the approval of the UC Davis Committee on Animal Research.
Micro-CT
A micro-CT (VivaCT 40; Scanco Medical, Bassersdorf, Switzerland) with an isotropic resolution of 10.5 μm, an X-ray source voltage of 70 kVp and a current of 145 μΑ was used to scan the right distal femur and the fourth lumbar vertebral body (LVB 4). For the distal femur, 200 slices were evaluated starting approximately 0.2 mm from the proximal end of the growth plate including a total metaphysis tissue volume of 2 mm3 for each specimen. The scans for the LVB 4 were initiated in the sagittal plane of the vertebral body and covered the entire cortical and trabecular bone. For each specimen, 200 slices were evaluated in the sagittal plane, and to compensate for growth plate irregularities in the axial plane, the secondary spongiosa was selected for analysis. A matching cube three-dimensional segmentation algorithm was used to separate mineralised bone from the bone marrow for grayscale images of the structure of mineralised tissue. Three-dimensional trabecular structural parameters were measured directly and methods used for calculation have been previously described [20]. A normalised index bone volume fraction (BV/TV) was used to compare samples of varying sizes. The methods for used for trabecular thickness (Tb.Th), trabecular separation (Tb.Sp) and trabecular number (Tb.N) have been previously described [20], [21], [22].
Histology for osteonecrosis
Both the right and left femurs were fixed in 10% formalin for 48 hours. The specimens were decalcified in 5% ethylene diamine tetra acetic acid (pH 7.2), and the solution was changed every 2 days until complete decalcification. The samples were processed and embedded in paraffin. Five-micron thick sagittal sections were stained with haematoxylin and eosin and evaluated for ON pathology by bright field microscopy at 20× magnification. Using the criteria by Yang et al, ON was detected in the distal femoral epiphysis (DFE) and required the presence of all three features in the DFE: empty lacunae, pyknotic osteocyte nuclei in the bone trabeculae and bone marrow and stromal necrosis [23], [24], [25], [26]. ON was evaluated by three independent blind observers (GM, DK, DIL).
Bone marrow fat quantitation
In each mouse, both distal femoral epiphyses were surveyed to establish the general level of marrow fat. A single representative field that represented the general appearance of the epiphyses was selected from one of the two epiphyses. Marrow fat was identified as sharply delineated spaces within the bone marrow cavity. The total area of the field was traced. Marrow fat areas and total area of the field were traced with Osteomeasure (Osteometrics, Inc.; Decatur, GA). Field total area was 0.70 ± 0.11 mm2. Fat volume/total volume was calculated for each mouse as 100 × (fat area/total area) [27].
Biomechanical testing
Mechanical properties of the tibia were determined by loading the left tibia to failure in three-point bending [28]. Tests were conducted in the posterior-to-anterior direction with a constant displacement rate of 0.05 mm/s, using a servo-hydraulic material testing machine (858 Mini Bionix II; MTS Systems, Eden Prairie, MN). Each tibia was loaded with the medial side of the bone in tension and the lateral side in compression. Load-displacement curves were analysed using MATLAB software (version R2008b; The Mathworks Inc., Natick, MA) to determine yield load, failure load, stiffness, work to fracture and post-yield displacement [29].
Whole-bone mechanical properties of the intact fifth lumbar vertebral body (LVB 5) were measured by compressing the vertebral body with a 3-mm diameter plate attached to a servo-hydraulic material testing machine (858 Mini Bionix II; MTS Systems, Eden Prairie, MN). In these compression tests, the cranial and caudal endplates of the caudal vertebrae were not altered before testing. Compression tests were conducted at a displacement rate of 0.05 mm/s [30].
Serum pro-collagen type 1N terminal propeptide level
Serum samples were collected at the time of sacrifice and stored at −80°C. Serum levels of pro-collagen type 1N terminal propeptide (P1NP) were measured using the Rat/Mouse P1NP EIA kit (Diagnostic Systems, Fountain Hills, AZ, USA). The assays were performed as recommended by the manufacturer, and all samples were assayed in duplicate. The coefficients of variations for interassay and intraassay measurements were <10% for all assays and are similar to the manufacturers' references [31], [32].
Tartrate-resistant acid phosphatase assay
Tartrate-resistant acid phosphatase (TRAP) staining of the distal femoral epiphyses was performed on a random sample from each of the treatment groups. The paraffin sections were stained for TRAP using the Sigma Acid Phosphatase Leukocyte Kit in accordance with the manufacturer's instructions. TRAP+ cells were defined by multinucleation and were considered mature osteoclasts when there were greater than three nuclei per cell and identified by a dark purple or red colour. The entire surface area of the epiphyses was examined with 20× magnification [33].
Statistical analysis
The group means and standard deviations were calculated for all outcome variables. The nonparametric Mann–Whitney U test was used to determine pairwise comparisons between the placebo and GC-treated groups within each strain. Differences were considered significant at p < 0.05 (GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA).
Results
Body weight
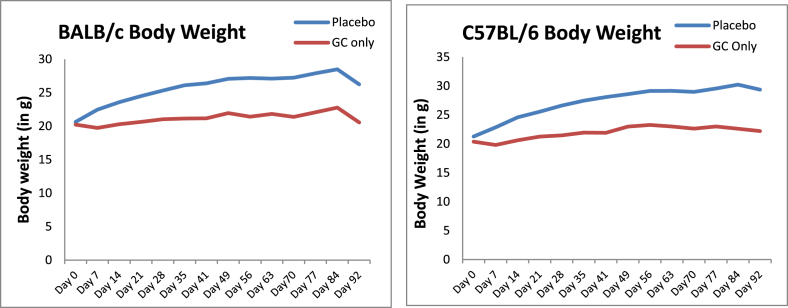
The body weights of GC-treated BALB/c and C57BL/6 mice were significantly lower compared to placebo from Day 7 to Day 90 (Figure 1, p < 0.05).
Figure 1.
Body weight from baseline of BALB/c and C57BL/6 mice. The body weight of GC-treated animals was significantly lower compared to the placebo group animals.
GC = glucocorticoid.
Trabecular bone mass and microarchitecture
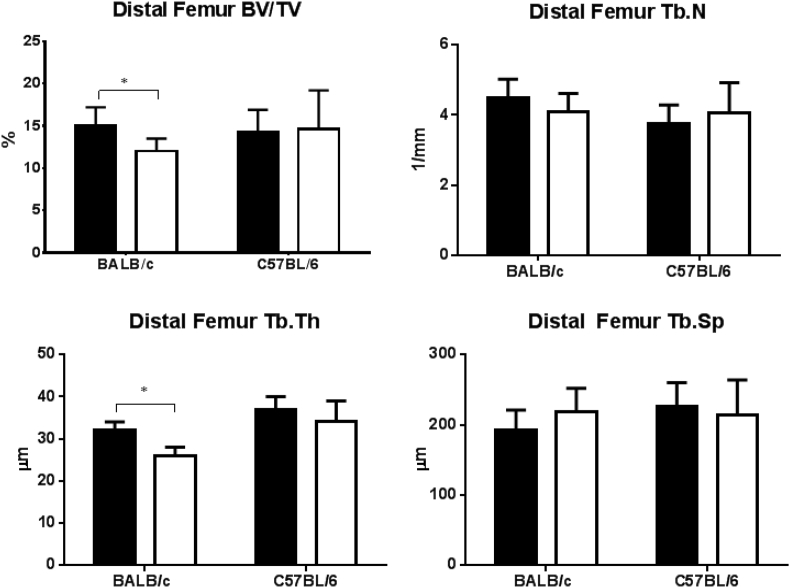
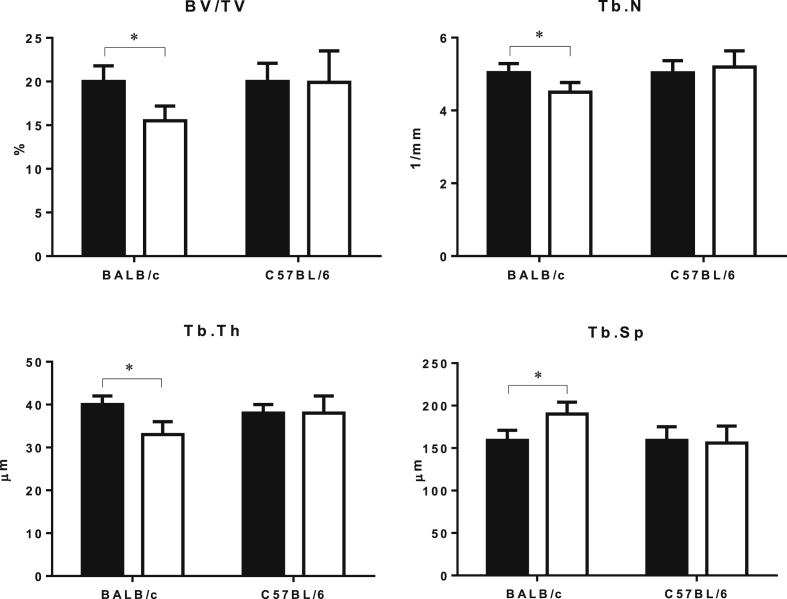
In the GC-treated BALB/c mice, the trabecular BV/TV and Tb.Th of the right distal femur were significantly lower compared to the placebo group (Figure 2, p < 0.05). In this mouse strain, there was reduced Tb.N and increased Tb.Sp in the GC-treated mice compared to the placebo. We did not find any significant changes for trabecular bone mass or microarchitecture in the GC-treated C57BL/6 mice compared to the placebo group (Figure 2). Analysis of the fourth lumbar vertebral body (LVB 4) found that in the GC-treated BALB/c mice, the trabecular BV/TV, Tb.N and Tb.Th were significantly reduced, and Tb.Sp was significantly increased compared to the placebo group (Figure 3, p < 0.05). There were no significant changes in any of the trabecular bone mass or microarchitecture parameters in GC-treated C57BL/6 mice compared to the placebo group (Figure 3).
Figure 2.
Effect of GC treatment on bone structures of the distal femoral metaphysis by micro-CT in BALB/c and C57BL/6 mice. Data are presented as mean ± SD, *p < 0.05 versus PL within the same strain.
BV/TV = bone volume fraction; GC = glucocorticoid; PL = placebo; SD = standard deviation; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular separation; black = placebo, white = GC-treated groups.
Figure 3.
Effect of GC treatment on trabecular bone structure in the lumbar vertebrae assessed by micro-CT. Data are presented as mean ± SD, *p < 0.05 versus PL within the same strain.
BV/TV = bone volume fraction; GC = glucocorticoid; PL = placebo; SD = standard deviation; Tb.N = trabecular number, Tb.Th = trabecular thickness, Tb.Sp = trabecular separation; black = placebo; white = GC-treated groups.
Osteonecrosis histopathology
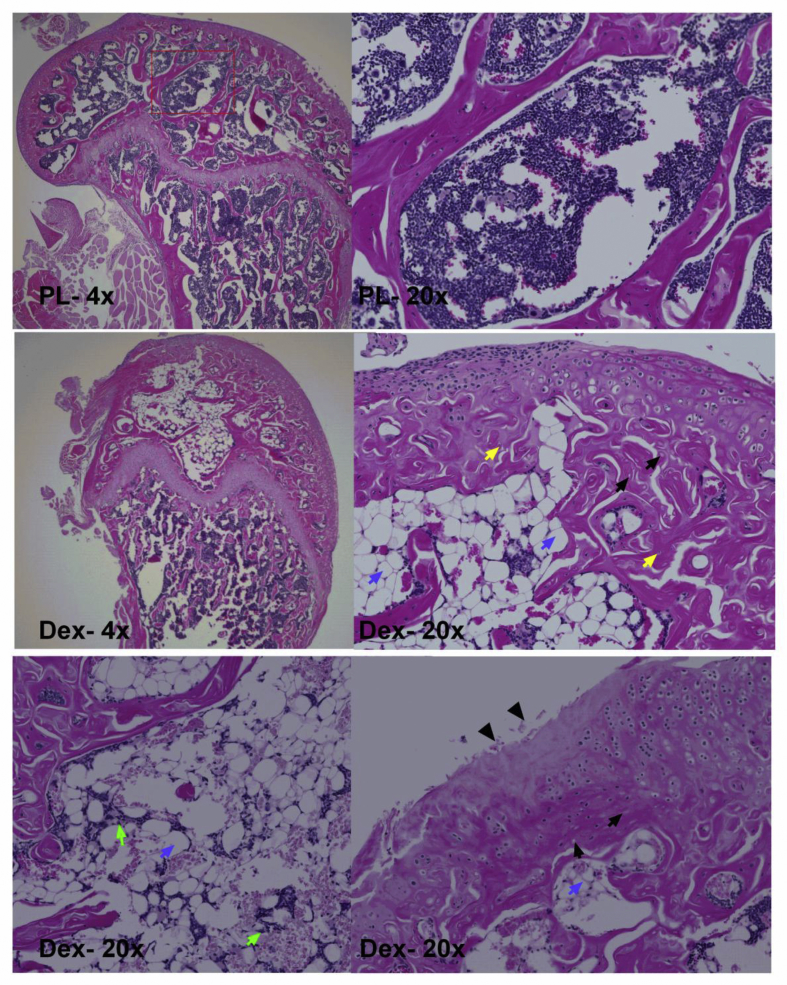
GC-induced ON lesions were observed in the DFE in 47% of the BALB/c mice and 25% of the C57BL/6 mice (Table 1). ON lesions were not observed in the placebo group. Histopathologic evaluation of the DFE in BALB/c mice showed less trabecular bone with empty osteocyte lacunae and pyknotic osteocyte nuclei (Table 1) with few osteoblasts lining the trabecular bone surfaces (Figure 4). Furthermore, an increased number of enlarged adipocytes, excessive adipocytes in the bone marrow, cartilage degradation and presence of fibrin thrombi in the blood vessels were also observed. In the GC-treated mice, there was a decrease in the number of haematopoietic cells in the medullary space of the DFE (Figure 4). We did not observe any such changes in the placebo group in which there were few empty lacunae and smaller adipocytes (Figure 4).
Table 1.
The total incidence of osteonecrosis of the distal femoral epiphysis in BALB/c and C57BL/6 male mice.
| Mouse strain | Empty osteocyte lacunae | Pyknotic nuclei of ghost osteocytes | Necrosis of marrow and stromal elements | Total incidence of ON |
|---|---|---|---|---|
| BALB/c | 13/18a | 13/18 | 9/18 | 0.47 |
| C57BL/6 | 9/15 | 8/15 | 5/15 | 0.25 |
ON = osteonecrosis.
Data values are given as number of mice/total number of mice.
Figure 4.
Representative histopathological sections of the distal femoral epiphysis in BALB/c mice treated with placebo (PL) or dexamethasone (GC). In PL-treated group, no ON lesions were found, with a few marrow fat cells and some sinusoids being observed in bone marrow. In GC-treated mice, most of the bone marrow space were either occupied by fat (blue arrows), developed necrotic bone marrow (green arrows) and trabeculae necrosis, which was characterised by empty lacunae (black arrows), pyknotic nuclei of osteocytes (yellow arrows) and surrounded by necrotic fat debris. haematoxylin and eosin staining; original magnifications 4× and 20×.
GC = glucocorticoid; ON = osteonecrosis.
Bone marrow fat quantitation
In the GC-treated BALB/c mice, bone marrow fat was significantly increased compared to the placebo group (p < 0.05). We also observed significant changes in bone marrow fat in the GC-treated C57BL/6 mice compared to the placebo group (p < 0.05). Also, there appeared to be a greater percentage of marrow fat in GC-treated BALB/c compared to GC-treated C57BL/6 mice.
Biomechanical testing
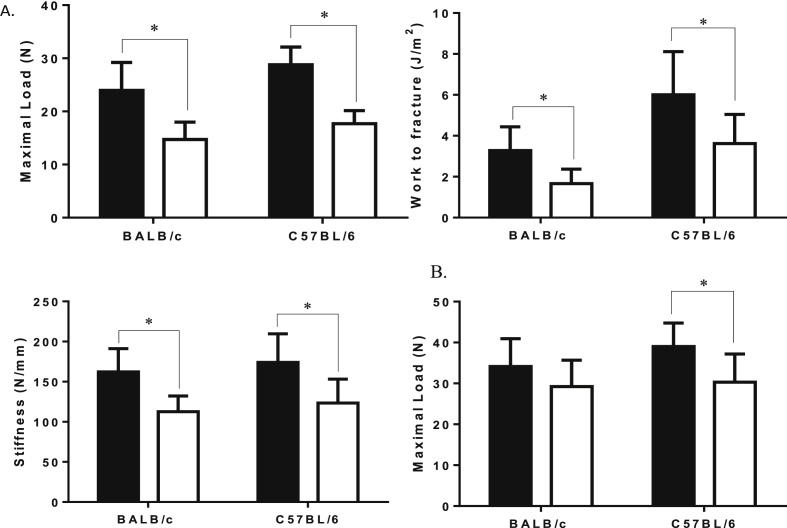
In the GC-treated BALB/c and C57BL/6 mice, the maximum load, work to fracture and whole bone stiffness of tibia were significantly reduced compared to the respective placebo groups (Figure 5A, p < 0.05). In the GC-treated C57BL/6 mice, maximum load of the LVB 5 was significantly reduced compared to the placebo group (Figure 5B). We did not observe significant changes in the maximal load of the LVB 5 in the GC-treated BALB/c mice compared to the placebo group.
Figure 5.
Mechanical tests of the tibia and lumbar vertebrae. (A) Maximal load, work to fracture and stiffness of the tibia (measured by three-point bend testing); (B) maximal load of the lumbar vertebral body (measured by compression test) is shown. GC treatment significantly decreased the maximum load, work to fracture and stiffness of tibia in the BALB/c and C57BL/6 strains compared to placebo. GC treatment significantly decreased maximal load in the C57BL/6 mice. Data are presented as mean ± SD, *p < 0.05 versus PL within the same strain.
GC = glucocorticoid; PL = placebo; SD = standard deviation; black = placebo; white = GC-treated groups.
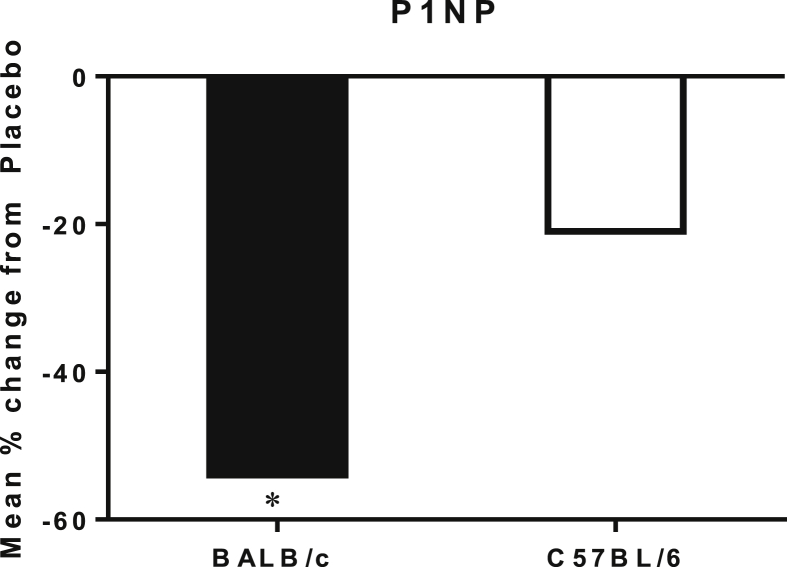
Serum P1NP level
At Day 90, serum P1NP levels of GC-treated BALB/c mice were significantly reduced compared to the placebo group (Figure 6, p < 0.05). We did not observe significant changes in serum P1NP in the GC-treated C57BL/6 mice compared to the placebo group.
Figure 6.
Percentage change in serum P1NP levels from placebo of BALB/c and C57BL/6 mice. The bone formation marker P1NP was significantly lower in the BALB/c strain compared to its placebo group. *p < 0.05 versus PL within the same strain.
PL = placebo.
TRAP assay
There were no significant differences in the TRAP+ cell count for osteoclast number in the GC-treated BALB/c and C57BL/6 mice compared to their respective placebo groups.
Discussion
In this study, we examined the susceptibility of GC-induced ON and bone loss by treating seven-week-old male BALB/c and C57BL/6 mice with oral dexamethasone. Prolonged treatment of GC for 90 days induced trabecular bone loss in the BALB/c mice but not in C57BL/6. Maximum load measured at the trabecular bone site of the tibia was decreased in both the BALB/c and C57BL/6 mice. Moreover, we observed the incidence of ON of 47% and 25% in the GC-treated BALB/c mice and C57BL/6 mice, respectively. The study results suggest that the male BALB/c mouse strain is a model for osteoporosis and ON following prolonged exposure to GCs.
Various animal studies have been performed to induce GC-induced ON and have reported variable results for the incidence of ON and development of bone loss. Rabbits have been used to try to develop a GC-induced ON model by treating the animals with varied doses and treatment intervals of intramuscular GC injections and have reported inconsistent results for the incidence of ON following GC treatment [16], [17]. Previous studies have used rabbit models to study the GC effects on bone after 4 weeks of treatment and reported significant decreases in bone mineral density and in the bone microarchitecture parameters of the GC-treated group compared to the control group [34]. Since the focus of the present study was to assess development of bone loss over a prolonged treatment period for clinical relevance in patients with chronic inflammatory disease receiving treatment with GCs, we used a mouse model to assess the incidence of ON and development of bone loss over a longer treatment period of 90 days.
Rats have also been used to try to develop a GC-induced ON model by treating the animals with intramuscular injections of methylprednisolone doses for varied time periods and have reported inconsistent results of the incidence of ON [35], [36]. This may have clinical relevance as the proximal femur is a frequent site for GC-induced ON in patients and can lead to a collapse of the femoral head that requires hip replacement surgery [5], [37], [38]; however, when rat models were used to study the effects of GCs on bone, it has been reported that treatment with prednisolone prevented rats from reduced bone mass and bone strength [39]. Furthermore, it has also been reported that GC-treated rats had a paradoxical increase in cancellous bone mass [19]. Since the objective of the present study was to assess the development of bone loss and GC-induced ON following a prolonged GC treatment period for clinical relevance in patients with chronic inflammatory disease receiving treatment with GCs, we used a mouse model to assess strain-specific differences of the incidence of ON and development of bone loss over a longer treatment period of 90 days in two different strains of male mice.
Previous mouse studies of GC-induced ON have reported the incidence of ON in male BALB/c mice. Janke et al treated two male BALB/c sub-strains of mice (BALBcJ and BALBcAnN) with an age range of 24–28 days with 4 mg/L or 8 mg/L dexamethasone in drinking water and once or twice weekly intraperitoneal injections of 7500 IU/kg asparaginase. They reported a 34% incidence of ON in BALBcJ male mice and a 16% incidence of ON in BALBcAnN male mice in the DFE 6 weeks after treatment [24]. In a previous study, Yang et al treated 14 mouse strains with varying doses of oral dexamethasone in drinking water for 15 weeks, an intraperitoneal injection of methylprednisolone and high-fat diets [23]. They observed ON only in the BALB/c mice and C57BL/6 mice treated with oral dexamethasone and reported a 40% incidence of ON in the distal femur in 4-week-old male BALB/cJ mice treated with 4 mg/L dexamethasone in drinking water for 12 weeks. They also reported that GC-induced ON was more prevalent in the male mice compared to the female mice [23]. Therefore, for a female mouse model, more mice may be required to develop GC-induced ON with a comparable incidence of ON. We used a similar treatment as used by Yang et al of oral dexamethasone in daily drinking water for 90 days because it is palatable and easily administered for a longer treatment period [23], [25]. Our results are comparable as we successfully showed GC-induced ON in male BALB/c and C57BL/6 mice with 47% and 25% incidence of ON, respectively. We chose to induce GC-induced ON in the BALB/c and C57BL/6 mice strains because it was previously reported that these strains were more susceptible to GC-induced ON compared to other strains in the study by Yang et al. Also, Harizi et al reported that the sensitivity to GC treatment of an inflammatory response was different between these strains, with GC sensitivity highest in C57BL/6 and lowest in BALB/c mice [40]. In addition, sensitivity to the GC receptor ligand for lymphocyte proliferation as an inflammatory response mechanism was highest in C57BL/6 [41]. These findings show interstrain immunologic differences and suggest that there may be an underlying genetic difference between these two mice strains. In this current study, we observed losses in bone mass and bone strength and reduced bone formation in the male BALB/c mice following 90 days of GC treatment, which were not reported previously by Yang et al. Also, the ON lesions had similar pathology to clinical ON including the presence of bone marrow necrosis, empty osteocyte lacunae, pyknotic osteocyte nuclei and enlarged adipocytes (Table 1) [17].
Additional characterisation of the ON phenotype included the evaluation of epiphyseal bone marrow fat, and we observed the GC-treated BALB/c mice bone marrow fat levels were significantly increased compared to the placebo group. The increase in the volume bone marrow fat was correlated with ON observed in the BALB/c mice compared to the C57BL/6 mice. Other investigators have reported an association with increased bone marrow fat and ON [42], [43].
Another important feature of GC-induced ON is decreased bone vascularity. Janke et al reported that in a high percentage of the BALB/c mice that developed ON lesions, there was evidence of changes within thrombi within the vessel lumen and changes within the vessel walls that they suggested might have contributed to the decrease in vascularity [24]. GC treatment has been shown to reduce bone vascularity and to reduce the volume of water present in the skeleton [15]. The reduction in blood flow and hydration of bone in the presence of GCs may be associated with the reduction in bone strength that is observed in both GC-treated animals and humans [4]. We observed a reduction in trabecular bone strength in GC-treated animals [44]. However, since bone strength is a composite of both bone mass and bone quality [45], [46], we may have just altered bone mass, but we did not have other variables measuring bone quality, which might explain bone strength as independent of bone mass [47].
BALB/c mice have increased susceptibility in developing GC-induced ON and bone loss [23], [48]. Compared to other mouse strains, BALB/c have been shown to have fewer native collateral blood vessels in the hind limb [49], and thus GC treatment may reduce the collateral circulation in BALB/c mice which could result in impaired blood flow in the bone. Furthermore, the microvascular anatomy of the subchondral zone in conjunction with reduced blood flow and embolisation due to hyperlipidaemia may explain the high incidence of ON observed in the distal femur of BALB/c mice, a feature that was not observed in other GC-induced ON animal models [50]. McLaughlin et al studied the GC effects on bone by treating 7-month-old female BALB/c mice with daily intraperitoneal injections of 10 mg/kg dexamethasone for 3 weeks and reported reduced bone mass in the distal femur and lumbar vertebrae [48]. In our current study, we observed comparable changes in the bone microarchitecture in the male BALB/c mice, and we showed the changes in the BALB/c mice compared to the C57BL/6 mice strain, which was not previously reported by McLaughlin et al. In addition, we reported development of bone loss following GC treatment over a longer treatment period of 90 days, which is clinically relevant to the patients receiving long-term GCs for chronic inflammatory diseases.
Weinstein et al recently developed a validated GC-induced ON model by treating 3-month-old and 7-month-old male C57BL/6 mice with 2.1 mg/kg/day of prednisolone pellets in 14-day intervals for 90 days. After 14 days of treatment, magnetic resonance imaging of the proximal femur revealed femoral head oedema and histology of the proximal femur had a significant reduction in bone vascularity, decreased hypoxia inducible factor 1 alpha and vascular endothelial growth factor expression and reduced bone strength of the femur. At Day 42 of GC exposure, both femoral head bone strength and bone volume fraction were reduced [51]. We have similar observation in the C57BL/6 strain that we did not detect bone loss, but observed bone strength was lower after 90 days of oral dexamethasone treatment. Since the focus of our study was to use histology to detect the incidence of GC-induced ON in the distal femoral epiphysis of male BALB/c mice compared to C57BL/6 mice, we will have yet to perform additional studies to confirm if we could detect early ON changes by magnetic resonance imaging in the femoral head of the C57BL/6 male mice prior to histologic changes [51].
The bone loss observed in our study could be attributed to the direct effect of GC on osteoclasts and osteoblasts [52]. We have previously demonstrated in male Swiss Webster mice that prednisolone treatment up to 56 days resulted in a significant bone loss, increased serum collagen type 1C-terminal telopeptide (CTX-I) level and decreased serum osteocalcin levels [22]. After 7 days of GC treatment, there was an upregulation of genes involved in osteoclast activation, function and adipogenesis. After 28 days and 56 days of GC treatment, the expression of genes associated with osteoblast activation and maturation was decreased, while the expression of Wnt antagonists such as DKK-1, sclerostin and WNF 1 was increased. In addition, after 28 days of GC treatment, the expression of genes associated with bone matrix degradation peaked [22]. Other mouse studies of GC effects on bone metabolism have reported increased receptor activator of nuclear factor kappa-Β ligand (RANKL)/osteoprotegerin (OPG) ratio which promotes osteoclastogenesis and leads to increased osteoclast activity and bone loss [53], [54]. After 90 days of GC treatment, we did not observe differences from the placebo-treated mice in TRAP staining.
The present study has several strengths including validated methods to assess ON lesions using histology, bone volume fraction using micro-CT and a comparison between two mouse strains; however, there are also some weaknesses. First, we only used one dose of dexamethasone, so we cannot know if there were more effective doses to induce GC-induced ON and bone loss. Second, we did not perform a formal evaluation of bone resorption either by bone turnover markers or by surface-based bone histomorphometry because the GC effects on bone resorption are present in the first few days of exposure, and the focus of this study was on the long-term effects of GC, on the effects on bone mass and the development of ON. Third, we evaluated ON lesions at one time point at Day 90, and we may have missed the progression of the development of ON lesions throughout the study. Finally, to characterise the ON phenotype, evaluation of cell apoptosis may have been useful. However, after 90 days of GC treatment, there is very little bone cell activity; therefore, we would have to perform these measurements at an earlier time point [19], [55]. While our study did not evaluate bone cell viability in the presence of GCs, other investigators have reported a dose dependent increase in GC-induced osteocyte and osteoblast apoptosis [14], [19], [55].
In conclusion, GC-induced ON and development of bone loss were more prevalent in the male BALB/c mice compared to the male C57BL/6 mice. Therefore, we recommend the use of male BALB/c mice for both diagnostic and therapeutic studies for the prevention and treatment of ON and bone loss.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement/Funding
This work was supported by NIH/NIAMS grant P50 AR063043, R01 AR043052 (NEL) and California Institute of Regenerative Medicine (CIRM), and the endowment for Aging Research at U. C. Davis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.07.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.van der Goes M.C., Jacobs J.W., Bijlsma J.W. The value of glucocorticoid co-therapy in different rheumatic diseases–positive and adverse effects. Arthritis Res Ther. 2014;16(Suppl 2):S2. doi: 10.1186/ar4686. PubMed PMID: 25608693; PubMed Central PMCID: PMCPMC4249491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lems W.F. Glucocorticoids: bad or safe for the bones? RMD Open. 2015;1(Suppl 1) doi: 10.1136/rmdopen-2015-000050. PubMed PMID: 26557373; PubMed Central PMCID: PMCPMC4632153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo K.H., Kim R., Kim Y.S., Ahn I.O., Cho S.H., Song H.R. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21(4):299–303. doi: 10.1007/s100670200078. PubMed PMID: 12189457. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein R.S. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010;46(3):564–570. doi: 10.1016/j.bone.2009.06.030. PubMed PMID: 19591965; PubMed Central PMCID: PMCPMC2823999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanatullah D.F., Strauss E.J., Di Cesare P.E. Current management options for osteonecrosis of the femoral head: part 1, diagnosis and nonoperative management. Am J Orthop (Belle Mead NJ) 2011;40(9):E186–E192. PubMed PMID: 22022684. [PubMed] [Google Scholar]
- 6.Xie X.H., Wang X.L., Yang H.L., Zhao D.W., Qin L. Steroid-associated osteonecrosis: Epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview) J Orthop Transl. 2015;3(2):58–70. doi: 10.1016/j.jot.2014.12.002. PubMed PMID: WOS:000215881400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millikan P.D., Karas V., Wellman S.S. Treatment of osteonecrosis of the femoral head with vascularized bone grafting. Curr Rev Musculoskelet Med. 2015;8(3):252–259. doi: 10.1007/s12178-015-9285-8. PubMed PMID: 26068178; PubMed Central PMCID: PMCPMC4596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mankin H.J. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326(22):1473–1479. doi: 10.1056/NEJM199205283262206. PubMed PMID: 1574093. [DOI] [PubMed] [Google Scholar]
- 9.Kerachian M.A., Seguin C., Harvey E.J. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114(3–5):121–128. doi: 10.1016/j.jsbmb.2009.02.007. PubMed PMID: 19429441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson E.O., Soultanis K., Soucacos P.N. Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthop Clin North Am. 2004;35(3):285–291. doi: 10.1016/j.ocl.2004.03.002. PubMed PMID: 15271536. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein R.S., Nicholas R.W., Manolagas S.C. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85(8):2907–2912. doi: 10.1210/jcem.85.8.6714. PubMed PMID: 10946902. [DOI] [PubMed] [Google Scholar]
- 12.Fowler T.W., Acevedo C., Mazur C.M., Hall-Glenn F., Fields A.J., Bale H.A. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Sci Rep. 2017;7:44618. doi: 10.1038/srep44618. PubMed PMID: 28327602; PubMed Central PMCID: PMCPMC5361115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng H., Zhang G., Wang Y.X., Yeung D.K., Griffith J.F., Leung K.S. Functional perfusion MRI predicts later occurrence of steroid-associated osteonecrosis: an experimental study in rabbits. J Orthop Res. 2009;27(6):742–747. doi: 10.1002/jor.20765. PubMed PMID: 19026010. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145(4):1835–1841. doi: 10.1210/en.2003-0990. PubMed PMID: 14691012. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein R.S., Wan C., Liu Q., Wang Y., Almeida M., O'Brien C.A. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9(2):147–161. doi: 10.1111/j.1474-9726.2009.00545.x. PubMed PMID: 20047574; PubMed Central PMCID: PMCPMC2858771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabata T., Kubo T., Matsumoto T., Hirata T., Fujioka M., Takahashi K.A. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology (Oxford) 2005;44(10):1233–1237. doi: 10.1093/rheumatology/keh721. PubMed PMID: 15972352. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T., Irisa T., Sugioka Y., Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40(11):2055–2064. doi: 10.1002/art.1780401119. PubMed PMID: 9365096. [DOI] [PubMed] [Google Scholar]
- 18.King C.S., Weir E.C., Gundberg C.W., Fox J., Insogna K.L. Effects of continuous glucocorticoid infusion on bone metabolism in the rat. Calcif Tissue Int. 1996;59(3):184–191. doi: 10.1007/s002239900107. PubMed PMID: 8694896. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–282. doi: 10.1172/JCI2799. PubMed PMID: 9664068; PubMed Central PMCID: PMCPMC508885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinney J.H., Haupt D.L., Balooch M., Ladd A.J., Ryaby J.T., Lane N.E. Three-dimensional morphometry of the L6 vertebra in the ovariectomized rat model of osteoporosis: biomechanical implications. J Bone Miner Res. 2000;15(10):1981–1991. doi: 10.1359/jbmr.2000.15.10.1981. PubMed PMID: 11028451. [DOI] [PubMed] [Google Scholar]
- 21.Lane N.E., Yao W., Balooch M., Nalla R.K., Balooch G., Habelitz S. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Min Res. 2006;21(3):466–476. doi: 10.1359/JBMR.051103. PubMed PMID: WOS:000235645100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao W., Cheng Z.Q., Pham A., Busse C., Zimmermann E.A., Ritchie R.O. Glucocorticoid-induced bone loss in mice can Be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 2008;58(11):3485–3497. doi: 10.1002/art.23954. PubMed PMID: WOS:000261039800025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L., Boyd K., Kaste S.C., Kamdem Kamdem L., Rahija R.J., Relling M.V. A mouse model for glucocorticoid-induced osteonecrosis: effect of a steroid holiday. J Orthop Res. 2009;27(2):169–175. doi: 10.1002/jor.20733. PubMed PMID: 18683891; PubMed Central PMCID: PMCPMC2718787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janke L.J., Liu C., Vogel P., Kawedia J., Boyd K.L., Funk A.J. Primary epiphyseal arteriopathy in a mouse model of steroid-induced osteonecrosis. Am J Pathol. 2013;183(1):19–25. doi: 10.1016/j.ajpath.2013.03.004. PubMed PMID: 23673001; PubMed Central PMCID: PMCPMC3702740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawedia J.D., Janke L., Funk A.J., Ramsey L.B., Liu C.C., Jenkins D. Substrain-specific differences in survival and osteonecrosis incidence in a mouse model. Comparative Med. 2012;62(6):466–471. PubMed PMID: WOS:000312684300002. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C., Janke L.J., Kawedia J.D., Ramsey L.B., Cai X., Mattano L.A., Jr. Asparaginase potentiates glucocorticoid-induced osteonecrosis in a mouse model. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151433. PubMed PMID: 26967741; PubMed Central PMCID: PMCPMC4788417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justesen J., Stenderup K., Ebbesen E.N., Mosekilde L., Steiniche T., Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. PubMed PMID: 11708718. [DOI] [PubMed] [Google Scholar]
- 28.Jepsen K.J., Pennington D.E., Lee Y.L., Warman M., Nadeau J. Bone brittleness varies with genetic background in A/J and C57BL/6J inbred mice. J Bone Miner Res. 2001;16(10):1854–1862. doi: 10.1359/jbmr.2001.16.10.1854. PubMed PMID: 11585350. [DOI] [PubMed] [Google Scholar]
- 29.Aslam M.N., Jepsen K.J., Khoury B., Graf K.H., Varani J. Bone structure and function in male C57BL/6 mice: effects of a high-fat Western-style diet with or without trace minerals. Bone Rep. 2016;5:141–149. doi: 10.1016/j.bonr.2016.05.002. PubMed PMID: 27350956; PubMed Central PMCID: PMCPMC4920365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tommasini S.M., Wearne S.L., Hof P.R., Jepsen K.J. Percolation theory relates corticocancellous architecture to mechanical function in vertebrae of inbred mouse strains. Bone. 2008;42(4):743–750. doi: 10.1016/j.bone.2007.12.009. PubMed PMID: 18258502; PubMed Central PMCID: PMCPMC2650241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao W., Cheng Z., Pham A., Busse C., Zimmermann E.A., Ritchie R.O. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 2008;58(11):3485–3497. doi: 10.1002/art.23954. Epub 2008/11/01, PubMed PMID: 18975341; PubMed Central PMCID: PMC2597521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Z., Wang R., Ang L.W., Yuan J.M., Koh W.P. Bone turnover biomarkers and risk of osteoporotic hip fracture in an Asian population. Bone. 2016;83:171–177. doi: 10.1016/j.bone.2015.11.005. PubMed PMID: 26555636; PubMed Central PMCID: PMCPMC4724247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao W., Dai W., Shahnazari M., Pham A., Chen Z., Chen H. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011410. PubMed PMID: 20625385; PubMed Central PMCID: PMCPMC2895664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin T., Liu J., Yang S., Liu X., Feng X., Fu D. Relation between the development of osteoporosis and osteonecrosis following glucocorticoid in a rabbit model. Indian J Orthop. 2016;50(4):406–413. doi: 10.4103/0019-5413.185606. PubMed PMID: 27512223; PubMed Central PMCID: PMCPMC4964774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Y.L., Li Y.L., Huang C., Gao K., Weng X.S. Systemic application of teriparatide for steroid induced osteonecrosis in a rat model. BMC Musculoskel Dis. 2015;16 doi: 10.1186/s12891-015-0589-z. PubMed PMID: WOS:000357711800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okazaki S., Nishitani Y., Nagoya S., Kaya M., Yamashita T., Matsumoto H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology. 2009;48(3):227–232. doi: 10.1093/rheumatology/ken462. PubMed PMID: WOS:000263603500005. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein R.S. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41(2):183–190. doi: 10.1007/s12020-011-9580-0. PubMed PMID: 22169965; PubMed Central PMCID: PMCPMC3712793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sissons H.A., Nuovo M.A., Steiner G.C. Pathology of osteonecrosis of the femoral head. A review of experience at the Hospital for Joint Diseases, New York. Skeletal Radiol. 1992;21(4):229–238. doi: 10.1007/BF00243063. PubMed PMID: 1626289. [DOI] [PubMed] [Google Scholar]
- 39.Okazaki Y., Tsurukami H., Nishida S., Okimoto N., Aota S., Takeda S. Prednisolone prevents decreases in trabecular bone mass and strength by reducing bone resorption and bone formation defect in adjuvant-induced arthritic rats. Bone. 1998;23(4):353–360. doi: 10.1016/s8756-3282(98)00116-1. PubMed PMID: 9763147. [DOI] [PubMed] [Google Scholar]
- 40.Harizi H., Homo-Delarche F., Amrani A., Coulaud J., Mormede P. Marked genetic differences in the regulation of blood glucose under immune and restraint stress in mice reveals a wide range of corticosensitivity. J Neuroimmunol. 2007;189(1–2):59–68. doi: 10.1016/j.jneuroim.2007.06.019. PubMed PMID: 17658621. [DOI] [PubMed] [Google Scholar]
- 41.Harizi H., Mormede P., Corcuff J.B. Inter-strain differences in glucocorticoid and mineralocorticoid effects on macrophage and lymphocyte functions in mice. J Neuroimmunol. 2008;204(1–2):38–42. doi: 10.1016/j.jneuroim.2008.08.004. PubMed PMID: 18812251. [DOI] [PubMed] [Google Scholar]
- 42.Sheng H., Sheng C.J., Cheng X.Y., Zhang G., Lee K.M., Leung K.S. Pathomorphological changes of bone marrow adipocytes in process of steroid-associated osteonecrosis. Int J Clin Exp Patho. 2013;6(6):1046–1050. PubMed PMID: WOS:000324306400006. [PMC free article] [PubMed] [Google Scholar]
- 43.Yin L., Li Y.B., Wang Y.S. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119(7):581–588. PubMed PMID: 16620700. [PubMed] [Google Scholar]
- 44.Yao W., Cheng Z., Busse C., Pham A., Nakamura M.C., Lane N.E. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58(6):1674–1686. doi: 10.1002/art.23454. PubMed PMID: 18512788; PubMed Central PMCID: PMCPMC3892702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammann P., Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–S18. doi: 10.1007/s00198-002-1345-4. PubMed PMID: 12730800. [DOI] [PubMed] [Google Scholar]
- 46.Fonseca H., Moreira-Goncalves D., Coriolano H.J., Duarte J.A. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. doi: 10.1007/s40279-013-0100-7. PubMed PMID: 24092631. [DOI] [PubMed] [Google Scholar]
- 47.Lane N.E. An update on glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 2001;27(1):235–253. doi: 10.1016/s0889-857x(05)70196-4. PubMed PMID: 11285998. [DOI] [PubMed] [Google Scholar]
- 48.McLaughlin F., Mackintosh J., Hayes B.P., McLaren A., Uings I.J., Salmon P. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone. 2002;30(6):924–930. doi: 10.1016/s8756-3282(02)00737-8. PubMed PMID: 12052464. [DOI] [PubMed] [Google Scholar]
- 49.Helisch A., Wagner S., Khan N., Drinane M., Wolfram S., Heil M. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26(3):520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. PubMed PMID: 16397137. [DOI] [PubMed] [Google Scholar]
- 50.Fan C., Foster B.K., Wallace W.H., Xian C.J. Pathobiology and prevention of cancer chemotherapy-induced bone growth arrest, bone loss, and osteonecrosis. Curr Mol Med. 2011;11(2):140–151. doi: 10.2174/156652411794859223. PubMed PMID: 21342129. [DOI] [PubMed] [Google Scholar]
- 51.Weinstein R.S., Hogan E.A., Borrelli M.J., Liachenko S., O'Brien C.A., Manolagas S.C. The pathophysiological sequence of glucocorticoid-induced osteonecrosis of the femoral head in male mice. Endocrinology. 2017 doi: 10.1210/en.2017-00662. PubMed PMID: 28938402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverman S.L., Lane N.E. Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2009;7(1):23–26. doi: 10.1007/s11914-009-0005-4. PubMed PMID: 19239826. [DOI] [PubMed] [Google Scholar]
- 53.Kondo T., Kitazawa R., Yamaguchi A., Kitazawa S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J Cell Biochem. 2008;103(1):335–345. doi: 10.1002/jcb.21414. PubMed PMID: 17516544. [DOI] [PubMed] [Google Scholar]
- 54.Thiele S., Ziegler N., Tsourdi E., De Bosscher K., Tuckermann J.P., Hofbauer L.C. Selective glucocorticoid receptor modulation maintains bone mineral density in mice. J Bone Miner Res. 2012;27(11):2242–2250. doi: 10.1002/jbmr.1688. PubMed PMID: 22714558. [DOI] [PubMed] [Google Scholar]
- 55.Jia J., Yao W., Guan M., Dai W., Shahnazari M., Kar R. Glucocorticoid dose determines osteocyte cell fate. Faseb J. 2011;25(10):3366–3376. doi: 10.1096/fj.11-182519. PubMed PMID: 21705669; PubMed Central PMCID: PMCPMC3177583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.