Figure 5.
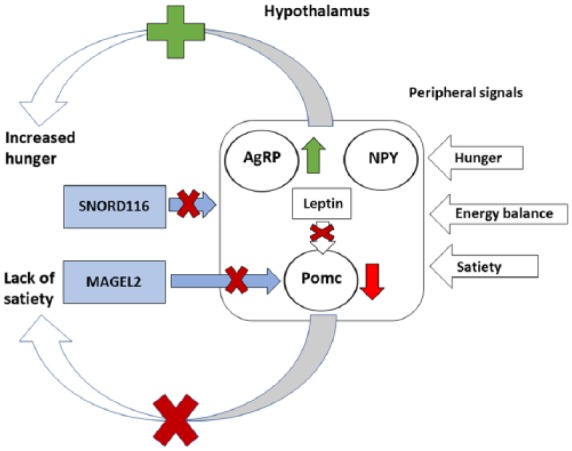
The dysregulation of the homeostatic feeding mechanism in PWS. Neural stimulation of neuropeptide Y (NPY)- and Agouti-like peptide (AgRP)-expressing neurons promotes feeding in response to peripheral signals of hunger, while neural stimulation of neurons expressing pro-opiomelanocortin (Pomc) inhibits feeding in response to peripheral signals of satiety. Long-term signals of energy balance such as leptin induce a fasting response and limit food intake when energy balance is favorable, preventing obesity. In PWS, lack of expression for SNORD116 may lead to neuronal degeneration and imbalance in the expression of AgRP and Pomc in the hypothalamus leading to increase in feeding. Similarly, MAGEL2 has been shown to be required for the leptin-mediated activation of Pomc-expressing neurons as MAGEL2 regulates the abundance of leptin receptors through ubiquitination pathways. Hence, both regulation of hunger and satiety and long-term regulation of energy balance are disrupted in Prader–Willi syndrome, leading to the impaired satiety and obesity typical of the syndrome.
