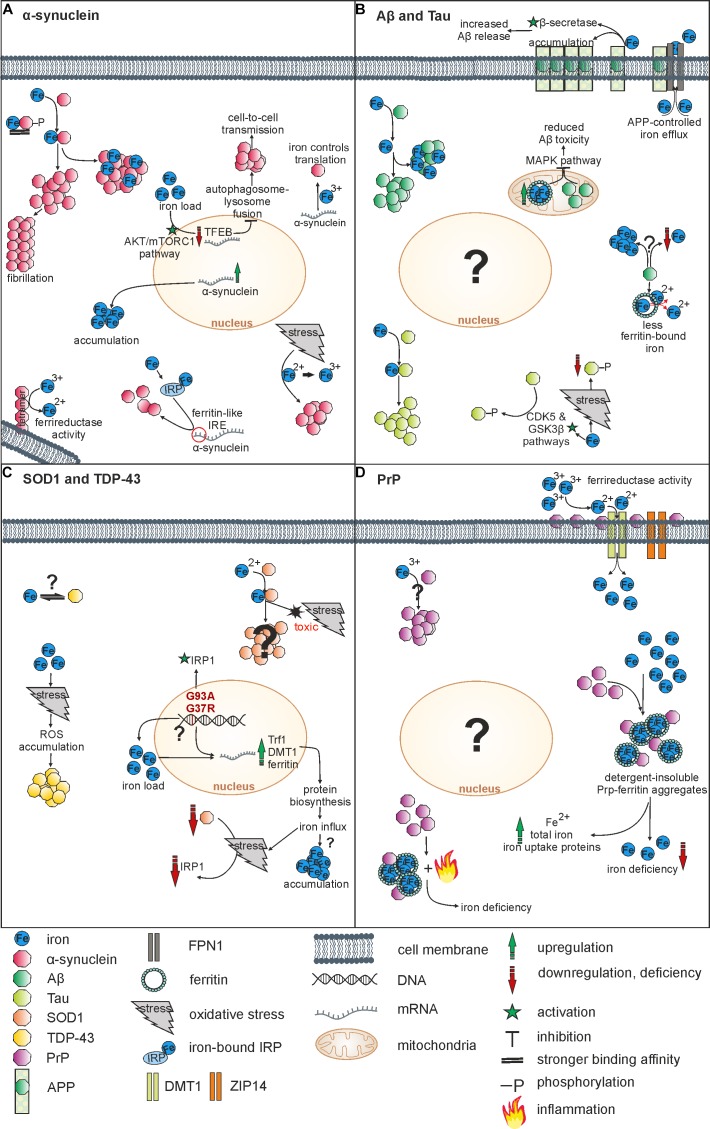
FIGURE 1.
Overview on relevant interactions of iron and NDDs-associated proteins. (A) Iron induces α-synuclein aggregation by direct binding or via oxidation. Indirectly, iron also influences α-synuclein on its transcriptional and translational level. α-synuclein acts as a ferrireductase and can induce iron accumulation by overexpression. (B) Iron fosters aggregation of both Aβ und Tau by binding. Whereas Aβ reduces levels of ferritin-bound iron, an overexpression of mitochondrial ferritin reduces Aβ toxicity. APP controls iron efflux and together with iron it affects the Aβ release. Furthermore, there is evidence for both, Aβ-induced iron accumulation and Aβ-induced iron depletion. Whereas iron increases Tau-phosphorylation via CDK5 and GSK3ß pathways, iron-induced oxidative stress reduces Tau-phosphorylation. (C) Iron binds SOD1, inducing oxidative stress and toxicity. Mutations of SOD1 lead to an upregulation of iron metabolism proteins followed by iron influx. Iron is suggested to affect TDP-43 aggregation indirectly via oxidative stress-mediated ROS accumulation. An interaction of iron and TDP-43 has not been objectified so far. (D) PrP operates as a ferrireductase partner of ZIP14 and DMT1 increasing Fe3+ uptake. Furthermore, PrP-ferritin aggregates induce iron deficiency and an upregulation of total iron, Fe2+ and iron uptake proteins. Inflammation processes may contribute to iron deficiency. Vice versa, Fe3+ triggers PrP accumulation within the cell.