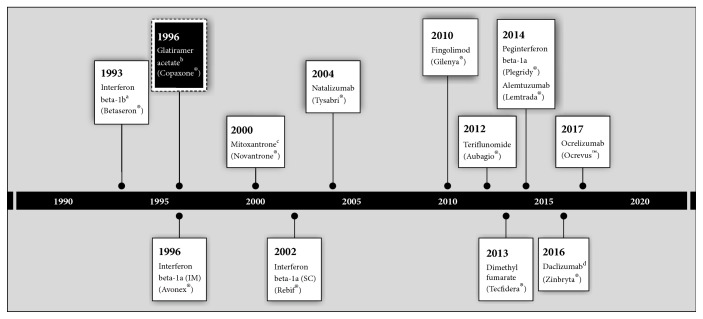
Figure 1.
Timeline of approval by the FDA of disease-modifying therapies for multiple sclerosis. FDA: Food and Drug Administration; IM: intramuscular; SC: subcutaneous. aInterferon beta-1b was also approved in 2009 as Extavia® (which is the Novartis-branded version of the Bayer product Betaseron®). bVarious generic versions of glatiramer acetate are in development. Glatopa™ [10] was approved in 2015 by the FDA. Other generic versions were approved in the EU in 2016 and by the FDA in 2017 [11, 12]. cBioequivalent generic mitoxantrone was approved in 2006. dSubsequently withdrawn (March 2018).