Abstract
Background:
25-Hydroxyvitamin D [25(OH)D] is a marker of nutritional status; however, chronic kidney disease (CKD) results in alterations in vitamin D metabolism, including the loss of vitamin D-binding proteins and alterations in CYP27B1 and CYP24 enzymes that metabolize 25(OH)D. This study was designed to determine the predictors of responsiveness to correction of vitamin D deficiency with oral vitamin D2 (ergocalciferol) in adults.
Methods:
A retrospective study of 183 veterans with 25(OH)D level <30 ng/mL, who were treated with 50,000 IU per week of vitamin D2, was performed. Logistic regression models were developed to determine the factors predicting the response to treatment, defined as either the change in serum 25(OH)D level/1000 IU of vitamin D2 or the number of vitamin D2 doses (50,000 IU per dose) administered.
Results:
The mean age of the patients was 63 ± 12 years. About 87% were men and 51% diabetic, and 29% had an estimated glomerular filtration rate of <60 mL/min/1.73 m2. The average number of vitamin D2 doses was 10.91 ± 5.95; the average increase in 25(OH)D level was 18 ± 10.80 ng/mL. 25(OH) D levels remained <30 ng/mL in 61 patients after treatment. A low estimated glomerular filtration rate and the presence of diabetes mellitus were significant independent predictors for inadequate response to vita-min D2 treatment in logistic regression models. Patients with CKD required greater amounts of vitamin D2 to achieve similar increases in 25(OH)D levels, versus non-CKD patients.
Conclusions:
The presence of CKD and diabetes mellitus is associated with resistance to correction of 25(OH)D deficiency with vitamin D2 therapy. The underlying mechanism needs to be evaluated in prospective studies.
Keywords: Vitamin D deficiency, Ergocalciferol, Chronic kidney disease, Vitamin D2, Resistance
Native vitamin D is a secosteroid that is available in the diet from either animal sources as cholecalciferol (vitamin D3) or plants, as ergocalciferol (vitamin D2).1 With ultraviolet light exposure, 7-dehydrocholesterol is endogenously converted in the skin to vitamin D3.2 Vitamin D3 is then hydroxylated in the liver by 25-hydroxylase to 25-hydroxyvitamin D [25(OH)D],1 that subsequently is converted to 1,25-dihydroxyvitamin D2 [1,25(OH)2D] by 1α-hydroxylase in the kidneys.3,4 This step is upregulated by parathyroid hormone (PTH) and downregulated by phosphate and fibroblast growth factor-23 (FGF23).5 25(OH) D and 1,25(OH)2D are metabolized by 24-hydroxylase to inactive metabolites, a step that is upregulated by FGF23 and downregulated by PTH.
Measurement of the serum level of 25(OH)D is considered to be the best biochemical index of vitamin D nutritional stores.6 Vitamin D deficiency is typically defined as a level <30 ng/mL.7 25(OH)D deficiency is a common problem in the general population, with a prevalence of 36% to 57%.8 Low circulating concentrations of 25(OH)D is even more prevalent in patients with chronic kidney disease (CKD), with a prevalence of 50% to 86%.9 Factors contributing to reduced levels of 25(OH)D in CKD include associated chronic illnesses, inadequate nutrition and lack of adequate sun exposure, similar to the general population. In addition, patients with CKD have more complex abnormalities of vitamin D metabolism that may contribute to reductions in 25(OH)D level that do not represent a true vitamin D deficiency. In this regard, patients with CKD may have nephrotic-range proteinuria that is associated with the loss of vitamin D-binding proteins (VDBPs) with subsequent 25(OH)D deficiency and resistance to vitamin D replacement therapy.10 In addition, elevated levels of FGF23 act as a vitamin D counterregulatory hormone by decreasing 1,25(OH)2D production through the downregulation of 1-α hydroxylase (encoded by CYP27B1) and the upregulation of 24-hydroxylase (encoded by CYP24).11,12 FGF23-mediated increments in CYP24 may also lead to reduced 25(OH)D levels and resistance to vitamin D replacement therapy by increasing the catabolism of 25(OH)D. This study was designed to evaluate the predictors of resistance to vitamin D2 (ergocalciferol) replacement therapy in a cohort of vitamin D-deficient patients who had a wide range of underlying kidney function, including patients with both CKD and normal kidney function.
METHODS
Study Population
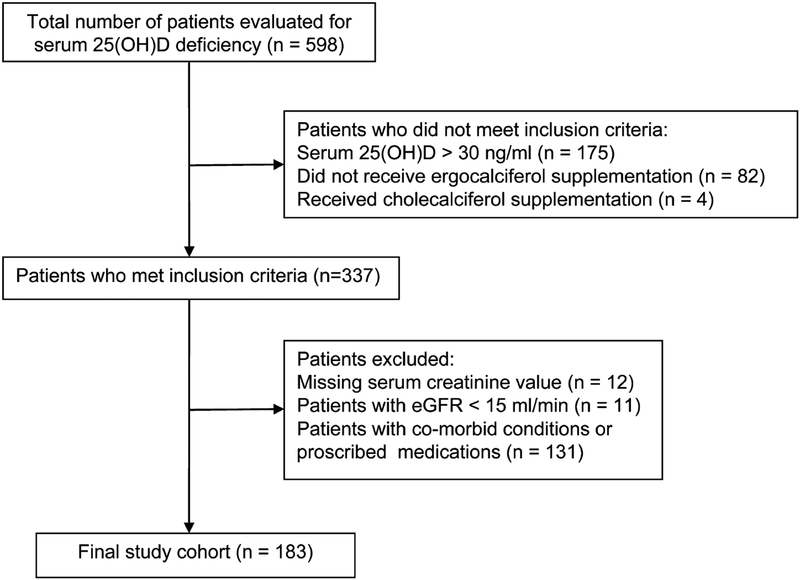
This is a retrospective study of veterans diagnosed with vitamin D deficiency [serum 25(OH)D level <30 ng/mL], between April 2009 and July 2010, who received vitamin D supplementation with vitamin D2 (ergocalciferol). The study was approved by the Veterans Affairs Medical Center Institutional Review Board (Memphis, TN). The inclusion criteria were as follows: (1) men and women over the age of 18 years, (2) serum 25(OH)D levels <30 ng/mL and (3) patients who received vitamin D2 replacement therapy. The exclusion criteria were as follows: (1) an estimated glomerular filtration rate (eGFR) of <15 mL/min/1.73 m2 and chronic dialysis; (2) cirrhosis, sarcoidosis, lymphoma, malabsorption syndrome, solid organ transplant or hyperparathyroidism; and (3) the chronic use of medications known to alter vitamin D metabolism, including rifampin, corticosteroids, antiepileptics, phosphate binders, active forms of vitamin D and calcimimetics. Initially, 598 patients with vitamin D deficiency were identified. Of these, 183 patients were included in the final analysis (Figure 1). The following information was obtained from the medical records: demographic data (age, sex and race), clinical characteristics (body weight, height, presence or absence of CKD, hypertension and diabetes mellitus [DM]), and the number of vitamin D2 doses. Treatment with vitamin D2 was confirmed by reviewing the pharmacy records for prescribing and releasing the medication to the patients. All patients received weekly doses of 50,000 IU of vitamin D2. The patient’s primary care provider determined the total number of vitamin D2 doses. Baseline laboratory data included the following: concentrations of serum creatinine, albumin, calcium, and 25 (OH)D, and urinary protein excretion. Serum 25(OH)D level was measured at baseline and at the end of the vitamin D2 treatment. Urinary protein excretion was measured as a ratio of spot urine protein-creatinine (UPC) ratio or as spot urine albumin-creatinine ratio (ACR). UPC ratios were converted to urine ACR by the following equation: ACR = UPC(1.054) ×0.596.13 All data used in the study were collected as part of usual patient care.
FIGURE 1.
Flow diagram for derivation of study cohort. 25(OH)D, 25-hydroxyvitaminD; eGFR, estimated glomerular filtration rate.
Procedures, Assays and Calculations
Response to vitamin D2 treatment was assessed as (1) the ratio of change in serum 25(OH)D level and the total amount of vitamin D2 received [final serum 25(OH)D concentration − baseline 25(OH)D concentration/1000 IU ergocalciferol], and (2) the total number of vitamin D2 doses (50,000 IU per dose) received by each patient. Serum 25 (OH)D levels were measured by immunochemiluminometric assay (ICMA) using the DiaSorin (Stillwater, MN) Liaison instrument in all samples, serum albumin levels by bromocresol green assay and serum creatinine levels by isotope dilution mass spectrometry traceable Jaffe method from Roche (Stillwater, MN). The eGFR was calculated according to the 4-variable Modified Diet in Renal Disease formula.14 CKD was defined as an eGFR <60 mL/min/1.73m2. Body mass index (BMI) was calculated by person’s weight in kilograms divided by his or her height in square meters.
Statistical Analysis
Continuous variables were presented as means and standard deviations, and categorical variables as percentages, unless otherwise specified. In this cohort, the median number of vitamin D2 doses was 10, which was used as the cutoff value between the high (>10) and low (≤10) number of vitamin D2 doses. The median number of vitamin D2 dose was used as the dependent variable in the first logistic regression model. The median value of the ratio of change in serum 25(OH)D level/1000 IU ergocalciferol dose was 0.03. This value was used as the cutoff value between the high (≥0.03) and low (<0.03) ratio, and was used as the dependent variable in the second logistic regression analysis. Impaired response (resistance) to vitamin D2 treatment was defined as the requirement for higher number of vitamin D2 dosages (>10 doses) or by a low ratio (<0.03) of change in 25(OH)D/1000 IU of vitamin D2. The following predictor variables were used for both logistic regression analyses: age; sex; race; BMI; DM; season during which 25(OH)D levels were obtained; baseline concentrations of albumin, calcium and 25(OH)D; and eGFR. A season variable (summer, winter) was created based on the timing of blood collection for baseline 25(OH)D, as sun exposure can affect previtamin D synthesis in human skin. The estimated odds ratio along with the corresponding 95% confidence intervals and P values are reported for all regression covariates. Only variables with a P value <0.10 were included in the multivariable stepwise logistic regression analysis. The final multivariable models were formally assessed for the presence of multicollinearity among the explanatory variables by using the variance inflation factor.15 Pretreatment and posttreatment laboratory test results were compared using paired t test and Wilcoxon signed-rank test. All tests were 2-sided, and a P value <0.05 was considered significant, unless otherwise stated. Statistical analysis was conducted using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Baseline Characteristics
Characteristics of the study cohort (n = 183) are depicted in Table 1. A total of 159 (87%) were men, 94 (48%) were African American, 93 (51%) were diabetic, 53 (29%) had an eGFR <60 mL/min/1.73 m2, 39 (21%) with stage 3 CKD and 14 (8%) with stage 4 CKD. The severity of serum 25(OH)D deficiency varied, with severe reductions (< 5 ng/mL) in 2 (1%), moderate reductions (5–15 ng/mL) in 79 (43%) and mild reductions (16–30 ng/mL) in 102 (56%) patients.
TABLE 1.
Baseline clinical characteristics of the study cohort
| Frequency | Mean ± SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age (yr) | 183 | 63 ± 12 | 31 | 98 |
| BMI (kg/m2) | 183 | 31 ± 7 | 19 | 69 |
| Initial serum creatinine (mg/dL) | 183 | 1.3 ± 0.6 | 0.6 | 3.7 |
| Initial eGFR (mL/min/1.73 m2) | 183 | 71.6 ± 29.5 | 20 | 140 |
| Initial serum 25(OH)D (ng/mL) | 183 | 16.8 ± 6.5 | 4.4 | 29.8 |
| No. ergocalciferol doses | 183 | 10.8 ± 5.9 | 2 | 36 |
| Initial serum calcium (mg/dL) | 169 | 9.3 ± 0.7 | 4.8 | 10.7 |
| Initial serum albumin (gm/dL) | 149 | 4.2 ± 0.4 | 2.4 | 5.1 |
| Urinary albumin creatinine ratio (g/g) | 133 | 0.2 ± 0.8 | 0 | 7.3 |
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Effects of Ergocalciferol Treatment
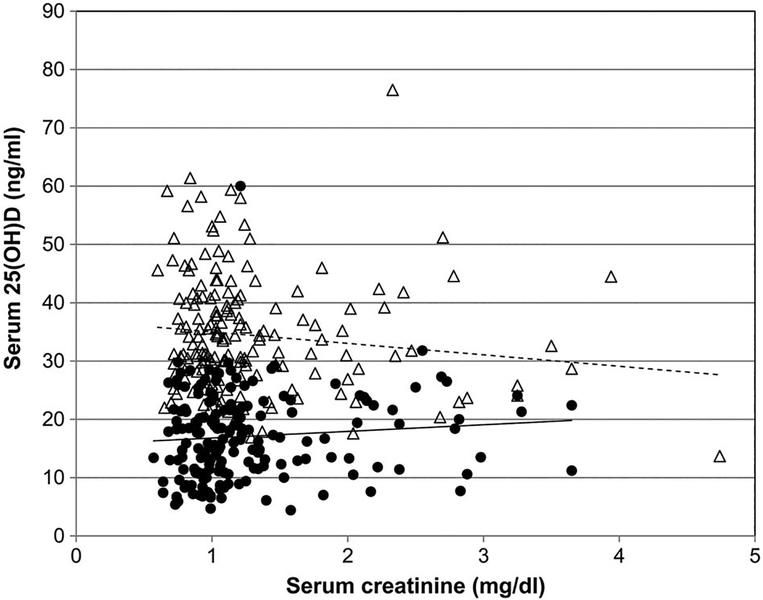
The average number of ergocalciferol doses administered was 10.91 ± 5.95, and the average duration of therapy was 2.5 months. Changes in concentrations of serum 25(OH)D, calcium, PTH and phosphorous levels are shown in Figure 2 and Table 2. The average increase in serum 25(OH)D level after vitamin D2 supplementation was 18.0 ± 10.8 ng/mL. In 61 patients (33%), serum 25(OH)D level remained below normal (,<30 ng/mL) after treatment. Baseline serum 25(OH)D concentrations correlated significantly with the female sex (r = 0.18, P = 0.01) and African American race (r = 0.25, P = 0.0007), but not with age, DM, baseline eGFR, winter season or the number of prescribed vitamin D2 dosages. Baseline eGFR inversely correlated with the number of vitamin D2 doses (r = 20.21, P = 0.003) and with patient’s age (r = 0.44, P = 0.0001), and positively correlated with the female sex (r = 0.17, P = 0.02) and serum albumin level (r = 0.16, P = 0.04). Despite similar baseline 25(OH)D concentrations, patients with CKD required significantly greater amounts of vitamin D2 (640 ± 296 versus 506 ± 291 thousand units, P 5 0.0006) to achieve similar increases in serum 25(OH)D levels, as compared with the non-CKD patients.
FIGURE 2.
Relationship between serum 25(OH)D and serum creatinine level showing pre- (●) and post (Δ) treatment 25(OH) D level. 25(OH)D, 25-hydroxyvitaminD.
TABLE 2.
Changes after treatment with ergocalciferol
| Frequency | Pretreatment | Posttreatment | Pa | |
|---|---|---|---|---|
| Serum 25(OH)D (ng/mL) | 183 | 17 ± 7 | 35 ± 10 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 172 | 72 ± 12 | 70 ± 25 | 0.06 |
| Serum calcium (mg/dL) | 140 | 9.3 ± 0.7 | 9.4 ± 0.5 | 0.001 |
| Serum PTH (pg/mL) | 30 | 114 ± 86 | 109 ± 75 | 0.76 |
| Serum phosphorus (mg/dL) | 19 | 3.8 ± 0.8 | 3.9 ± 0.8 | 0.95 |
| Serum albumin (gm/dL) | 113 | 4.2 ± 0.4 | 4.1 ± 0.4 | 0.002 |
Data expressed as mean ± standard deviation.
Variables eGFR and serum phosphorous were tested by paired t test; Wilcoxon signed-rank test used for the rest of the variables. 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
Resistance to Ergocalciferol Treatment
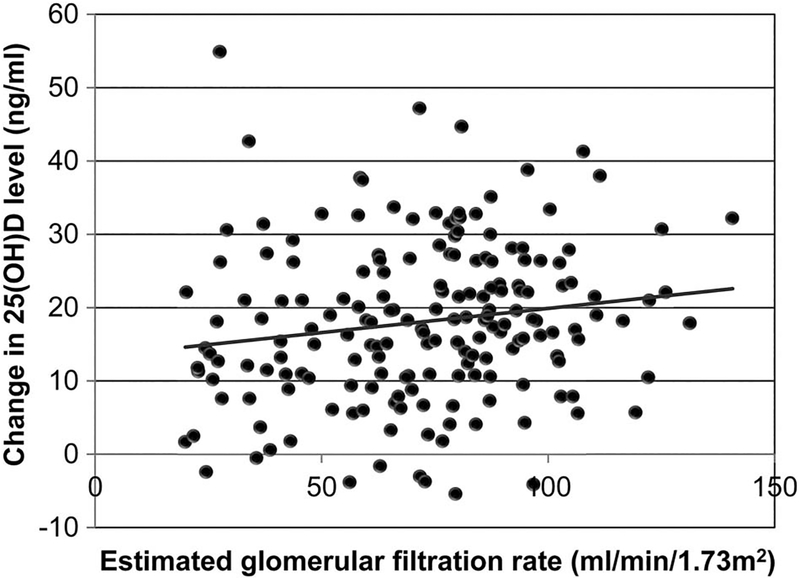
The change in serum 25(OH)D level after the ergocalciferol treatment correlated significantly with the eGFR (Figure 3). Univariate logistic regression analysis of the change in serum 25 (OH)D level/1000 IU of vitamin D2 showed statistically significant association of female sex, baseline eGFR, baseline serum 25(OH)D level and the presence of DM (Table 3). These 4 variables and the interaction terms between DM and baseline eGFR and baseline 25(OH)D were used to construct a multivariable model for the response to vitamin D2 supplementation. In the final multivariable model, lower baseline eGFR, higher baseline serum 25(OH)D level and the presence of DM without the interaction terms significantly predicted inadequate response to vitamin D2 supplementation (Table 3).
FIGURE 3.
Relationship between actual change in serum 25 (OH)D level and estimated glomerular filtration rate. 25(OH)D, 25-hydroxyvitaminD.
TABLE 3.
Predictors of an inadequate response to vitamin D supplementation defined by low ratio of change in serum 25(OH)D level/1000 IU ergocalciferol in univariate and multivariable analyses
| Univariate | Multivariablea | |||||
|---|---|---|---|---|---|---|
| Predictor variables | OR | 95% CI | P | ORb | 95% CI | P |
| Race (1 = white, 0 = black) | 0.63 | 0.35–1.12 | 0.12 | |||
| Age (yr) | 1.00 | 0.98–1.02 | 0.98 | |||
| Sex (1 = male, 0 = female) | 0.42 | 0.17–1.05 | 0.06 | |||
| BMI (kg/m2) | 1.01 | 0.97–1.06 | 0.56 | |||
| HTN (1 = presence, 0 = absence) | 1.18 | 0.60–2.34 | 0.63 | |||
| DM (1 = presence, 0 = absence) | 1.82 | 1.01–3.27 | 0.05 | 2.18 | 1.09–4.33 | 0.027 |
| Season (1 = winter, 0 = summer) | 0.91 | 0.50–1.65 | 0.75 | |||
| Baseline serum albumin (gm/dL) | 1.44 | 0.69–3.03 | 0.33 | |||
| Baseline serum calcium (mg/dL) | 0.99 | 0.63–1.58 | 0.98 | |||
| Baseline serum 25(OH)D (ng/mL) | 1.13 | 1.08–1.19 | <0.0001 | 1.14 | 1.08–1.21 | <0.0001 |
| Baseline eGFR (mL/min/1.73m2) | 0.97 | 0.96–0.99 | <0.0001 | 0.97 | 0.96–0.99 | <0.0001 |
Hosmer-Lemeshow goodness-of-fit statistics χ2(8) of 5.32 and P value 0.72 suggesting that the model fits our data well. Variance inflation factor: 1.04 for eGFR, 1.04 for DM and 1.04 for 25(OH)D.
Variable excluded (P > 0.10) during stepwise logistic regression modeling. 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate, HTN, hypertension; OR, odds ratio.
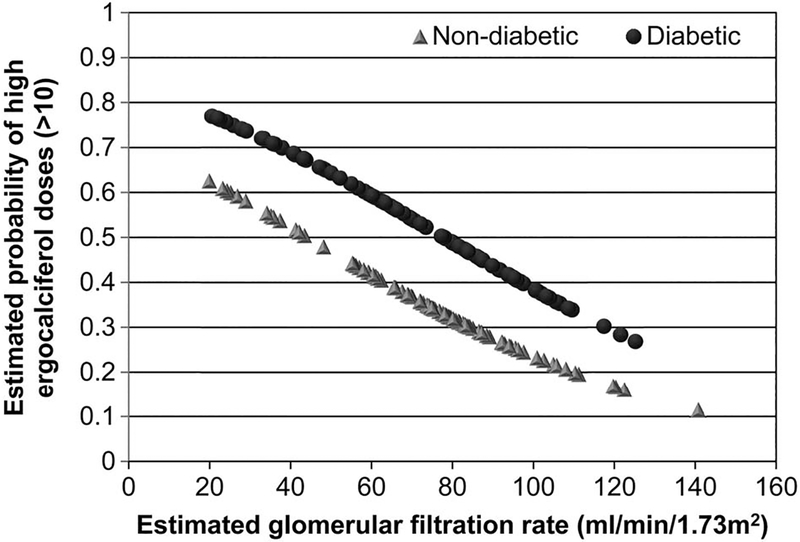
The response to vitamin D2 supplementation in relation to the number of ergocalciferol doses administered was evaluated separately. In univariate logistic regression analysis, the presence of DM, low baseline eGFR and white race significantly predicted >10 weekly doses of ergocalciferol (Table 4). Lower baseline eGFR and the presence of DM were found to be significant predictors of a higher number of vitamin D2 doses in the final multivariable model (Table 4). To help visualize analysis results, conditional effect plot for outcome prediction of high vitamin D2 dose was drawn on the basis of the fitted final logistic regression model (Figure 4). It plotted the estimated probability of having a high vitamin D2 dose (>10) against a chosen continuous covariate, eGFR, with the values of the other discrete and continuous covariates held in constant. The probabilities of resistance increased with worsening eGFR. The plot also showed that for a given value of eGFR, probabilities of resistance to vitamin D2 supplementation were higher in diabetic patients, as compared with nondiabetic patients.
TABLE 4.
Predictors of an inadequate response to vitamin D supplementation defined by >10 ergocalciferol doses in univariate and multivariable analyses
| Univariate | Multivariablea | |||||
|---|---|---|---|---|---|---|
| Predictor variables | OR | 95% CI | P | ORb | 95% CI | P |
| Race (1 = white, 0 = black) | 1.85 | 1.02–3.33 | 0.042 | |||
| Age (yr) | 1.00 | 0.98–1.02 | 0.81 | |||
| Sex (1 = male, 0 = female) | 0.95 | 0.40–2.26 | 0.91 | |||
| HTN (1 = presence, 0 = absence) | 1.23 | 0.62–2.45 | 0.55 | |||
| DM (1 = presence, 0 = absence) | 2.11 | 1.16–3.82 | 0.014 | 1.93 | 1.04–3.56 | 0.04 |
| BMI (kg/m2) | 0.99 | 0.96–1.04 | 0.97 | |||
| Season (1 = winter, 0 = summer) | 0.73 | 0.40–1.33 | 0.30 | |||
| Serum albumin (gm/dL) | 0.69 | 0.33–1.45 | 0.33 | |||
| Serum calcium (mg/dL) | 0.71 | 0.43–1.17 | 0.18 | |||
| Baseline serum 25(OH)D (ng/mL) | 1.03 | 0.96–1.08 | 0.19 | |||
| Baseline eGFR (mL/min/1.73 m2) | 0.98 | 0.97–0.99 | 0.0004 | 0.98 | 0.97–0.99 | 0.0009 |
Hosmer-Lemeshow goodness-of-fit statistics χ2(8) of 2.98 and P value 0.94 suggesting that the model fits our data well. Variance inflation factor: 1.015 for DM and 1.015 for eGFR.
Variable excluded (P >0.10) during stepwise logistic regression modeling.
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate, HTN, hypertension; OR, odds ratio.
FIGURE 4.
Conditional effect plot showing the relationship between the estimated probability of inadequate response to ergocalciferol treatment (ergocalciferol dose >10) and estimated glomerular filtration rate in diabetic and non-diabetic patients.
Urinary ACR was available in a subset of patients (n = 133). Thirteen patients had an ACR of >0.6 g/g and 3 patients had nephrotic-range proteinuria (ACR >.2.2 g/g). Urinary ACR failed to contribute to either of the final multivariable logistic regression models for the response to vitamin D2 supplementation.
After vitamin D2 supplementation, serum 25(OH)D level decreased in 9 patients. The eGFR was ≥60 mL/min/1.73 m2 in 6 of the 9 patients. A sensitivity analysis performed after excluding these patients showed the same predictors of inadequate response to vitamin D therapy.
DISCUSSION
In the current study, we evaluated the effectiveness of weekly vitamin D2 treatment to raise serum 25(OH)D levels in a cohort of vitamin D-deficient patients that included patients with both normal and impaired renal function (CKD stages 3 and 4). We found that DM and CKD were significant independent predictors of resistance to vitamin D replacement therapy. Response to vitamin D2 treatment showed progressive worsening with decreasing eGFR, and the effect was more pronounced in diabetic than in nondiabetic patients. The association of resistance to vitamin D supplementation with CKD and DM was independent of age, sex, ethnicity, seasonal variation, BMI and hypertension.
Despite similar baseline 25(OH)D levels, patients with CKD, as compared with non-CKD patients, required significantly greater amounts of ergocalciferol (640 ± 296 versus 506 ± 291 thousand units, P = 0.0006) to achieve similar increments in serum 25(OH)D levels. In the current study, the change in serum 25(OH)D level correlated significantly with eGFR (r = 0.25, P =0.0004), and baseline eGFR was inversely correlated with the number of vitamin D2 doses (r = 20.21, P = 0.003). Moreover, in this cohort, the presence of low baseline eGFR and DM was associated with higher probabilities of resistance to vitamin D therapy and for the requirement of high doses of vitamin D2 to achieve normal vitamin D levels (Figure 4).
To our knowledge this is the first study to identify the contribution of CKD and DM in a general group of patients with vitamin D deficiency. Previous studies that investigated possible resistance to treatment of 25(OH)D deficiency with vitamin D2, as evidenced by the difficulty in achieving target 25(OH)D levels, only included patients with CKD.16–18 The response was not compared to a group with normal renal function. Al-Aly et al17 treated vitamin D-deficient CKD patients with a vita-min D2 regimen according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative-recommended protocol (15 doses).19 After 6 months of follow-up, serum 25(OH) D level increased by an average of 16.6 ng/mL to 27.2 ng/mL. However, the increase in 25(OH)D level was <5 ng/mL in 45% of the patients. In another study by Zisman et al,16 vitamin D-deficient CKD stage 3 and 4 patients were treated according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative protocol. Serum 25(OH)D levels increased to a range of 31 to 35 ng/mL in 60% of patients. In contrast, 33% of patients in the current study had serum 25(OH)D levels <30 ng/mL after treatment. This was influenced by the presence of renal disease, in that 17% of the patients in the low eGFR group (eGFR <60 mL/min) and 8% patients in the normal eGFR group (eGFR >60 mL/min) had <5% increase in serum 25(OH)D level (P = 0.09). We observed a somewhat better response in patients with CKD in the current study compared with the prior study, which may be related to the higher number of vitamin D2 doses, because 40% of the patients with CKD in the current study received >15 vitamin D2 doses.
There is controversy as to whether vitamin D2 is less potent and effective than vitamin D3 in raising serum 25(OH) D levels in the general population and that vitamin D2 may enhance degradation of 25-hydroxyvitamin D3.20–22 The mechanism for these differences is not clear but could be secondary to the lower affinity of vitamin D2 to VDBPs, to differences in metabolism between vitamin D2 and vitaminD3, and to a shorter shelf life of vitamin D2.20To our knowledge, there have been no direct comparison studies of vita-min D2 (ergocalciferol) versus vitamin D3 (cholecalciferol) therapy for correction of vitamin D deficiency in patients with CKD.
The mechanism underlying the resistance to vitamin D2 in diabetics and CKD is not known. Inadequate intake, hypoalbuminemia and albuminuria have been implicated as possible reasons for low 25(OH)D levels in diabetic patients.23,24 There is no evidence that gastrointestinal absorption of 25(OH)D is altered in patients with CKD or diabetes.25 Vitamin D metabolites are transported in the blood bound to VDBPs (85%–88%) and albumin (12%–15%), with very little circulating in the free form.3,5,10 Because the liver produces VDBPs and albumin, and these proteins can be lost in the setting of nephrotic syndrome, these conditions can result in low levels of the transport proteins.26,27 This results in low total levels of vitamin D metabolites [25(OH)D and 1,25(OH)2D] without necessarily changing the free circulating levels.10,27
Albuminuria in diabetics has been associated with higher urinary VDBP excretion, as compared with the normal subjects,26 suggesting that higher requirements for vitamin D replacement therapy in albuminuric vitamin D-deficient CKD and diabetic patients may be related to the loss of VDBPs. In the current study, however, proteinuria per se was not associated with a diminished response to vitamin D replacement therapy in the subset of 133 of the 182 subjects in whom quantitative measurements for proteinuria were available. Lee et al28 reported a significant association between resistance to vitamin D supplementation and higher BMI. This study included a small number of patients (n 5 17) with an average BMI of 25 kg/m2 and the duration of cholecalciferol supplementation was also short. Although DM is strongly associated with obesity, we did not find any significant association between decreased response to ergocalciferol therapy and high BMI in the current study.
Other explanations for the increased vitamin D2 requirements may be related to complex derangements in vitamin D metabolism that occur in CKD, including PTH stimulation of CYP27B1,11,29,30 and FGF23 inhibition of CYP27B131–38 and stimulation of CYP24 expression.31–37,39 In an adenine-induced animal model of CKD, mice with CKD had elevated FGF23 and PTH levels and decreased 1,25(OH)2D levels with a 5-fold increase in the messenger RNA expression of CYP24 and a 2-fold increase in the messenger RNA expression of CYP27B1, as compared with control animals.38 The effects of FGF23 on CYP27B1/CYP24 in CKD, with subsequent enhanced degradation of 25 (OH)D by the CYP24 pathway, could potentially explain the decreased responsiveness to vitamin D replacement associated with low eGFR. In advanced CKD, extrarenal CYP24 may play a role in the degradation of 25(OH)D.24 At present, we have no data on either CYP24 activity in the kidney or catabolism of 25 (OH)D in diabetic patients with CKD. CYP24 expression in peripheral blood monocytes, however, was reported to be similar among patients with type 1 DM and healthy individuals.40 Further prospective studies are needed to evaluate whether increased vita-min D catabolism is associated with resistance to treatment with vitamin D therapy in CKD.
Limitations of the current study include the observational and cross-sectional nature of the study. Most patients included in this study were men and >60 years of age, which may limit generalizability. Measurements of quantitative proteinuria were available only in a subset of patients (133 of the 183 patients) and direct measurements of 1,25(OH)2D, intact PTH and FGF23 were not available. Vitamin D2 dosing was standardized (50,000 IU per week) in the study with pharmacy documentation that the medication was dispensed to the patient. Adherence to the medication, however, could not be further verified because of the retrospective study design. The total duration of ergocalciferol therapy was determined by the patients’ primary care providers and, therefore, was not standardized.
This retrospective cohort study provides evidence for resistance to vitamin D2 replacement therapy in vitamin D-deficient patients with CKD and DM. This may reflect underlying altered metabolism of vitamin D associated with these conditions. Prospective studies of vitamin D metabolism in vitamin D-deficient patients treated with nutritional vitamin D supplements are needed to define the underlying altered mechanism.
Footnotes
Presented in abstract form: “Evidence of Disordered Vitamin D Metabolism in Chronic Kidney Disease,” J Investig Med 2011;59:459A.
The authors have no financial or other conflicts of interest to disclose.
REFERENCES
- 1.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 2005;289:F8–28. [DOI] [PubMed] [Google Scholar]
- 2.Webb AR, Pilbeam C, Hanafin N, et al. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr 1990;51:1075–81. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D and the kidney. Kidney Int 1987;32:912–29. [DOI] [PubMed] [Google Scholar]
- 4.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999;96:507–15. [DOI] [PubMed] [Google Scholar]
- 5.Lips P Vitamin D physiology. Prog Biophys Mol Biol 2006;92:4–8. [DOI] [PubMed] [Google Scholar]
- 6.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insuf-ficiency. Lancet 1998;351:805–6. [DOI] [PubMed] [Google Scholar]
- 7.Looker AC, Johnson CL, Lacher DA, et al. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011;59:1–8. [PubMed] [Google Scholar]
- 8.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–73. [DOI] [PubMed] [Google Scholar]
- 9.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007;71:31–8. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD, Siiteri PK, Ryzen E, et al. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab 1985;61:969–75. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol 2007;18:1637–47. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429–35. [DOI] [PubMed] [Google Scholar]
- 13.Stoycheff N, Stevens LA, Schmid CH, et al. Nephrotic syndrome in diabetic kidney disease: an evaluation and update of the definition. Am J Kidney Dis 2009;54:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;1<30:461–70. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Price B. Regression analysis by example, 2nd ed. New York: John Wiley and Sons; 1991. p.191–2. [Google Scholar]
- 16.Zisman AL, Hristova M, Ho LT, et al. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 2007;27: 36–43. [DOI] [PubMed] [Google Scholar]
- 17.Al-Aly Z, Qazi RA, Gonzalez EA, et al. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 2007;50:59–68. [DOI] [PubMed] [Google Scholar]
- 18.Pesenson A, Masaki M. Prevalence of 25-OH vitamin D (25-OH) deficiency in patients with chronic kidney disease (CKD) and effects of correction following KDOQI guidelines [Abstract]. J Am Soc Nephrol 2006;(suppl 1). [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 suppl 3):S1–201. [PubMed] [Google Scholar]
- 20.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 2006;84:694–7. [DOI] [PubMed] [Google Scholar]
- 21.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89: 5387–91. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93: 677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int 1999;55:1019–27. [DOI] [PubMed] [Google Scholar]
- 24.de Boer IH, Ioannou GN, Kestenbaum B, et al. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2007;50: 69–77. [DOI] [PubMed] [Google Scholar]
- 25.Avioli LV, Birge S, Lee SW, et al. The metabolic fate of vitamin D3–3H in chronic renal failure. J Clin Invest 1968;47:2239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thrailkill KM, Jo CH, Cockrell GE, et al. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab 2011;96:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikle DD, Halloran BP, Gee E, et al. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest 1986;78:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P, Greenfield JR, Seibel MJ, et al. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med 2009;122:1056–60. [DOI] [PubMed] [Google Scholar]
- 29.Bajwa A, Forster MN, Maiti A, et al. Specific regulation of CYP27B1 and VDR in proximal versus distal renal cells. Arch Biochem Biophys 2008;477:33–42. [DOI] [PubMed] [Google Scholar]
- 30.Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D3–24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem 2003;88:234–7. [DOI] [PubMed] [Google Scholar]
- 31.Bai XY, Miao D, Goltzman D, et al. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 2003;278:9843–9. [DOI] [PubMed] [Google Scholar]
- 32.Evenepoel P, Meijers B, Viaene L, et al. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol 2010;5:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson T, Nisbeth U, Ljunggren O, et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 2003;64:2272–9. [DOI] [PubMed] [Google Scholar]
- 34.Beckman MJ, Tadikonda P, Werner E, et al. Human 25-hydroxyvitamin D3–24-hydroxylase, a multicatalytic enzyme. Biochemistry 1996;35: 8465–72. [DOI] [PubMed] [Google Scholar]
- 35.Masuda S, Byford V, Arabian A, et al. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology 2005;146:825–34. [DOI] [PubMed] [Google Scholar]
- 36.Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int 2010;78:463–72. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010;78:975–80. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 2007;117:4003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan B, Xing Y, Horst RL, et al. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in the hyp-mouse. Endocrinology 2004;145:3804–12. [DOI] [PubMed] [Google Scholar]
- 40.Ramos-Lopez E, Bruck P, Jansen T, et al. CYP2R1-, CYP27B1- and CYP24-mRNA expression in German type 1 diabetes patients. J Steroid Biochem Mol Biol 2007;103:807–10. [DOI] [PubMed] [Google Scholar]