Figure 7.
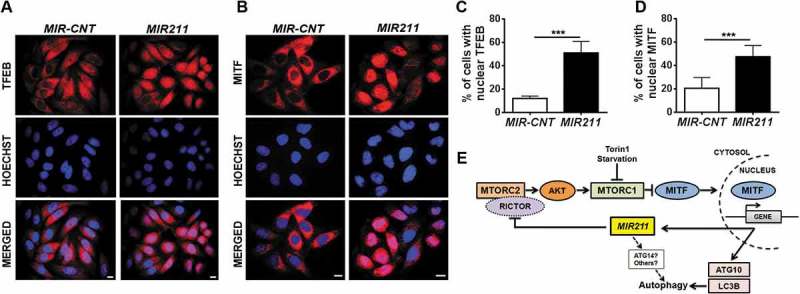
MIR211 overexpression led to MITF translocation to the nucleus. (a and b) Endogenous TFEB (A) or MITF (B) intracellular localizations were analyzed using indirect immunostaining with specific antibodies in HeLa cells transfected with MIR-CNT or MIR211. Scale bar: 10 µm. (c and d) Quantification of endogenous TFEB (c) or MITF (d) nuclear localization (mean± SD, n = 3 independent experiments, ***p < 0.01). (e) A model depicting the MITF-MIR211 autophagy feed-forward regulation pathway. Downregulation of RICTOR by MIR211 blocks MTORC2 activity, leading to AKT inhibition that is followed by MTORC1 blockage. Under these conditions, MITF that was sequestered in the cytosol migrates to the nucleus and contributes to the transactivation of autophagy-related genes as well as MIR211. Upregulation of the miRNA under these conditions creates a feed-forward loop that amplifies and sustains autophagy during stress. Although, we have shown here that RICTOR was a direct and rate-limiting target of MIR211 in autophagy control (see the rescue assays in Figure 5(j)), additional direct or indirect connections involving other MIR211 targets (e.g., ATG14) might also be contributing to the further amplification of the autophagic activity.
