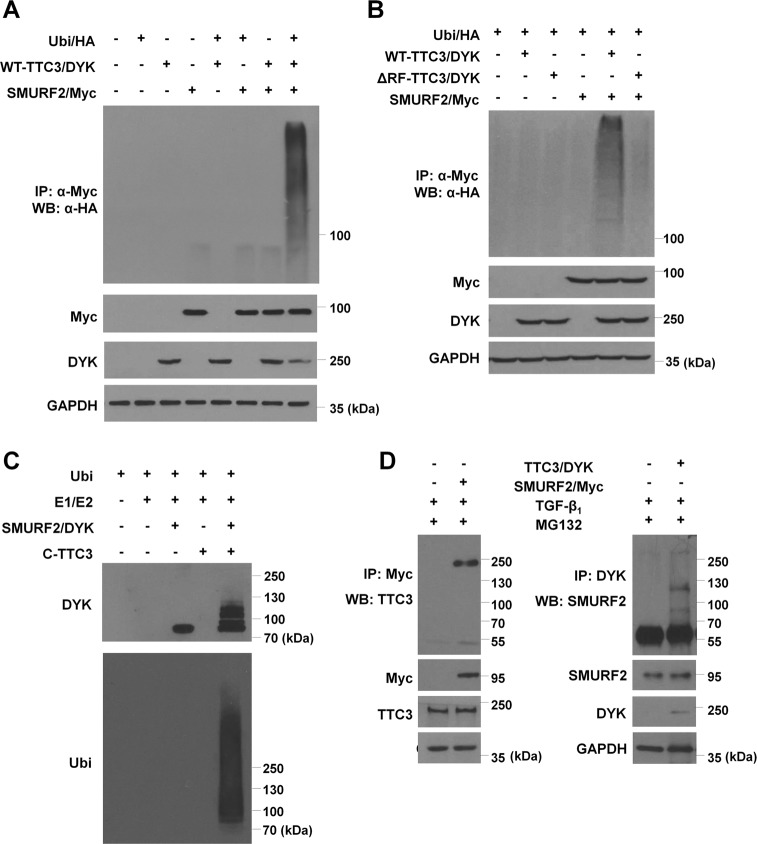
Fig. 3. TTC3-mediated SMURF2 ubiquitylation.
a TTC3-mediated SMURF2 ubiquitylation in HEK293 cells. HEK293 cells were transfected with TTC3/DYK plasmids on day 1 and transfected with SMURF2/Myc and Ubi/HA plasmids on day 2. After an overnight incubation, 5 μM MG132 was added and the cells were harvested on day 4. SMURF2/Myc was immunoprecipitated with anti-Myc antibody-conjugated agarose beads and ubiquitylated SMURF2 was detected with an anti-HA antibody. b Requirement of the RING domain of TTC3 for SMURF2 ubiquitylation in HEK293 cells. HEK293 cells were transfected with the wild-type TTC3 (WT-TTC3/DYK) and RING domain-deleted mutant TTC3 (ΔRF-TTC3/DDK), and the cell ubiquitylation assay was done as in (a). c TTC3-mediated SMURF2 ubiquitylation in vitro. Recombinant human SMURF2/DYK was incubated with 6×-His recombinant C-terminal wild-type TTC3 (WT-C-TTC3; Ile1540 to Arg2025) with human E1 (UBE1) and E2 (UBCE2E3) for 4 h at 30 °C. Ubiquitylated SMURF2/DYK was detected by western blotting using an anti-ubiquitin antibody. d Interaction between SMURF2 and TTC3 in BEAS-2B cells. BEAS-2B cells were transfected with either SMURF2/Myc or TTC3/DYK, and treated with 10 ng/ml TGF-β1 for 1 d. After cell lysis, SMURF2 and TTC3 were immunoprecipitated with anti-Myc or anti-DYK antibodies, respectively. Bound TTC3 and SMURF2 were detected by western blotting