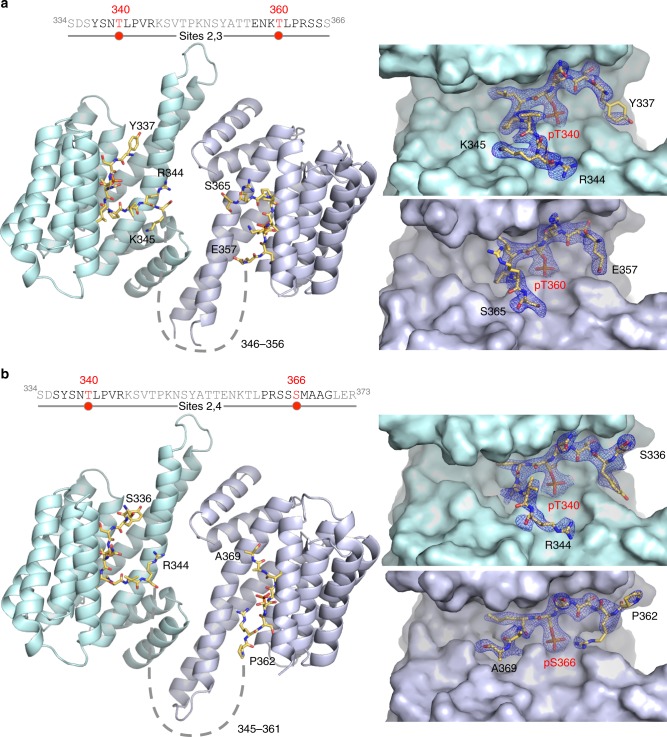
Fig. 4.
Structures of complexes of 14-3-3θ with doubly-phosphorylated IRSp53 peptides. a, b Complexes of 14-3-3θ with the Sites 2,3 and Sites 2,4 peptides, showing the overall structure (left) and close-ups of the binding pockets of 14-3-3θ and corresponding 2Fo–Fc electron density maps contoured at 1σ (right). In the sequence diagrams shown, the portions of the peptides observed in the structures are highlighted black and the phosphorylation sites are highlighted red, whereas unresolved amino acids are shown in gray. Dashed lines indicate the path between the observed portions of the bound peptides in the structures. Note that the sequences around the two phosphorylation sites of each peptide are different enough to be clearly distinguishable in the electron density maps in the separate pockets of 14-3-3θ (see also Supplementary Figure 7), as confirmed by cross-comparisons with the structures of the corresponding singly-phosphorylated peptides (Supplementary Figures 5 and 6)