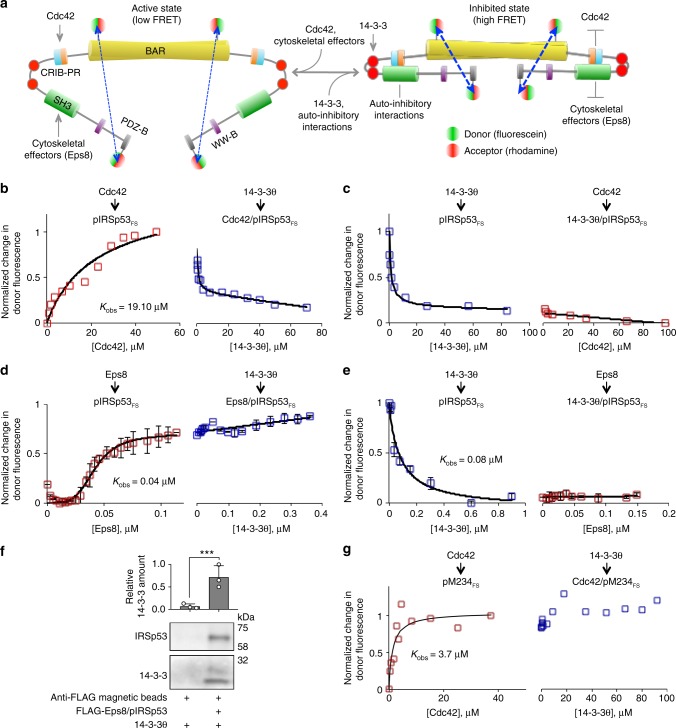
Fig. 5.
A FRET-sensor assay shows inhibition of pIRSp53 by 14-3-3. a FRET sensor assay designed to report on conformational changes occurring in pIRSp53 as a function of interactions with binding partners. Phosphorylated IRSp53 (mutant S519C) was expressed and purified as shown in Fig. 1 and labeled at C230 (within the BAR domain) and C519 (near the C-terminus) with the donor/acceptor pair fluorescein/rhodamine (pIRSp53FS). The same batch of probes and donor/acceptor ratio were used in all labeling reactions. Conformational changes in pIRSp53FS are detected based on the amount of FRET between the fluorescence probes at these two distant positions. b, c FRET titration of 2 μM GNPPNP-Cdc42 (G12V mutant) into 0.2 μM pIRSp53FS (red squares), followed by the titration of 0.2 μM 14-3-3θ (blue squares) and reverse-order titration. d, e FRET titration of 0.2 μM Eps8 into 0.05 μM pIRSp53FS (red squares), followed by the titration of 4 μM 14-3-3θ (blue squares) and reverse-order titrations. Pairs of titrations were normalized to the total change in FRET. Each titration was fit to a non-cooperative binding function (black lines), except that of Eps8 into pIRSp53FS, which was fit to a cooperative binding function with non-specific binding (see Methods). f Relative abundance of 14-3-3 pulling down with anti-Flag magnetic beads in the presence or the absence of the FLAG-Eps8/pIRSp53 complex. Error bars are ± s.d. from three independent experiments, shown as open circles. The statistical significance of the measurements was determined using an unpaired two-sided Student’s t-test (***p < 0.001). g FRET titration of 2 μM GNPPNP-Cdc42 into 0.2 μM mutant M234FS (red squares), followed by the titration of 0.2 μM 14-3-3θ (blue squares)