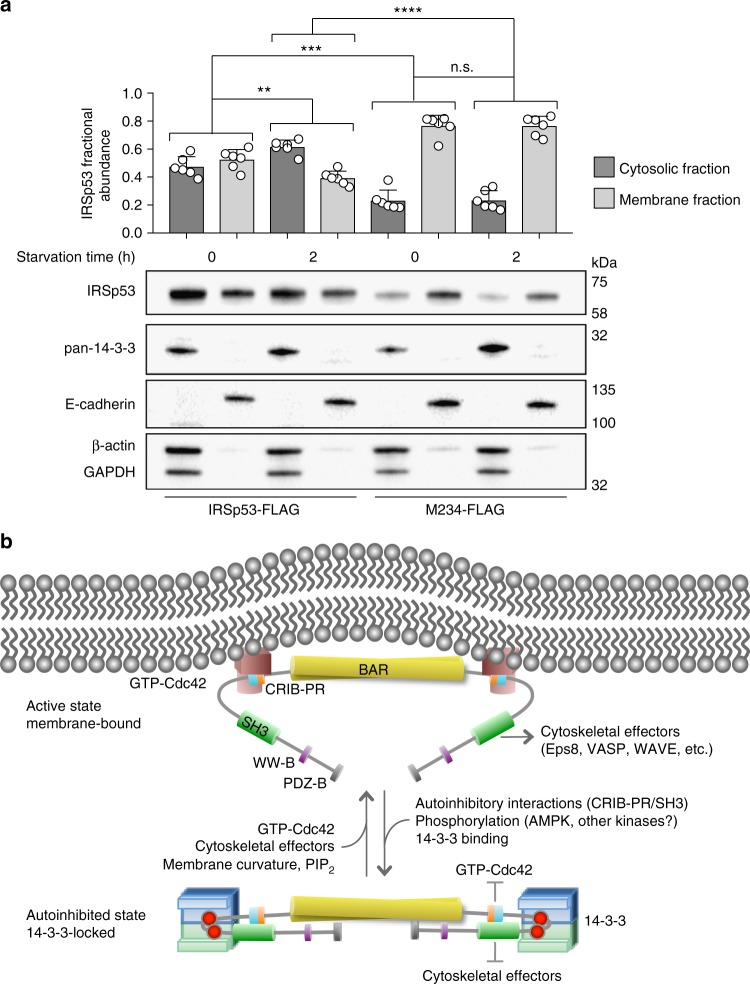
Fig. 6.
Mechanism of IRSp53 regulation by 14-3-3. a 14-3-3 inhibits membrane binding of pIRSp53. The relative abundances of WT IRSp53-FLAG and mutant M234-FLAG in the cytosolic (black bars) and membrane fractions (gray bars) of fed and serum-starved HEK293T cells, were determined by weighting the abundance of IRSp53 or M234 against the combined abundance of GAPDH and β-actin, for the cytosolic fraction, or E-cadherin, for the membrane fraction. Error bars are ± s.d. from three different transfections and two experiments per transfection (shown as open circles). The statistical significance of the measurements was determined using an unpaired two-sided Student’s t-test (n.s., non-significant; **p < 0.01; ***p < 0.001; ****p < 0.0001). b Model of IRSp53 activation and phosphorylation-dependent inhibition by 14-3-3. IRSp53 is activated by the binding of Cdc42 to the CRIB-PR domain and/or cytoskeletal effectors to the SH3 domain. Activation exposes the I-BAR domain for binding to the plasma membrane. Phosphorylation of IRSp53 and subsequent binding of 14-3-3 to phosphorylation sites located between the CRIB-PR and SH3 domains stabilizes the autoinhibited conformation and inhibits the binding of Cdc42 and downstream cytoskeletal effectors