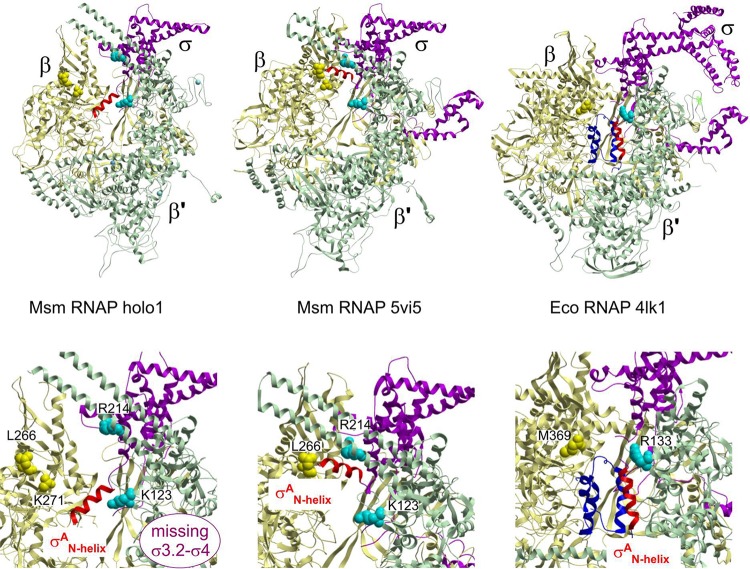
FIG 3.
The σAN-helix changes position within the RNAP. (Top left) The N-terminal α-helix (red) of σA (amino acids [aa] 149 to 162) is located within the primary channel of the M. smegmatis open-conformation holoenzyme RNAP. The σAN-helix is positioned between the β′ coiled-coil and the inner edge of β domain 2 (Table S2) and oriented perpendicular to the nonconserved domain (NCD) β′i1 finger. (Top middle) The N-terminal α-helix (red) of σA (aa 149 to 162) in the closed-conformation mycobacterial RNAP structures (12) is positioned on the periphery in between the β and σ′ subunit claws, parallel to the NCD β′i1 finger. (Top right) σ701.1 from E. coli in complex with RNAP positioned in the primary channel of RNAP (8). RNAP subunits are colored as follows: β, yellow; β′, green; α, gray; α′, cyan; ω, orange; σA/70, magenta; the N-terminal part of σA/70, dark blue; σAN-helix, red. (Bottom row) Detailed views of the respective σA/70N helix regions from the structures in the upper row.