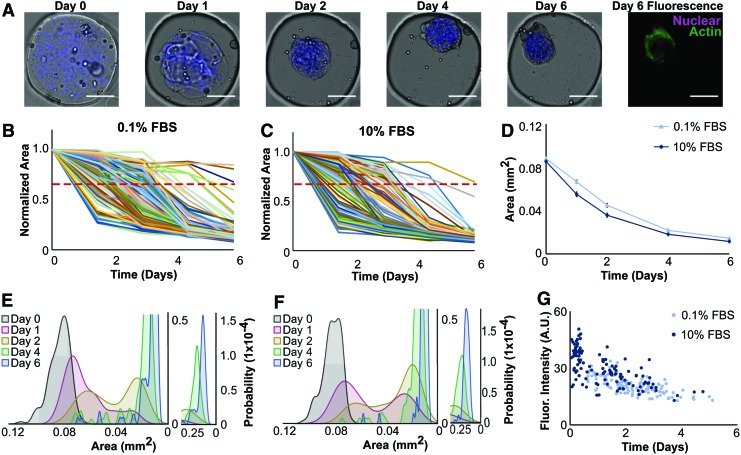
FIG. 4.
Microwells facilitate tracking of discrete microtissues and coupling live imaging data with endpoint staining. (A) Brightfield and fluorescence imaging of collagen microtissues containing NHLFs and encapsulated fluorescent beads (blue) to mark construct borders were cultured in 0.1% (or 10%) serum for 1 week. All scale bars 100 μm. (B, C) Areas of 121 individual microtissues cultured in 0.1% FBS (B) and 137 constructs cultured in 10% FBS (C) were measured and plotted. A red dashed line indicates a threshold compaction level of 30% compaction. Projected area is normalized to day 0 for each microtissue compaction trajectory. (D) Population averages show similar compaction trajectories between 0.1% and 10% serum conditions. Shown with standard error. (E, F) Probability density functions of microtissue area at each time point reveal heterogeneity in the populations, indicated by bimodal distributions on day 1 (red) and day 2 (yellow) populations. (G) We observed a strong negative correlation between actin intensity and the time at which a 30% reduction in projected area occurred, as indicated by correlation coefficients of −0.68 and −0.65 for 0.1% and 10% FBS conditions, respectively. This suggests that a faster compaction rate is correlated to increased actin expression. We also found that higher serum conditions corresponded to elevated actin intensity and increased compaction rates. FBS, fetal bovine serum. Color images available online at www.liebertpub.com/tec