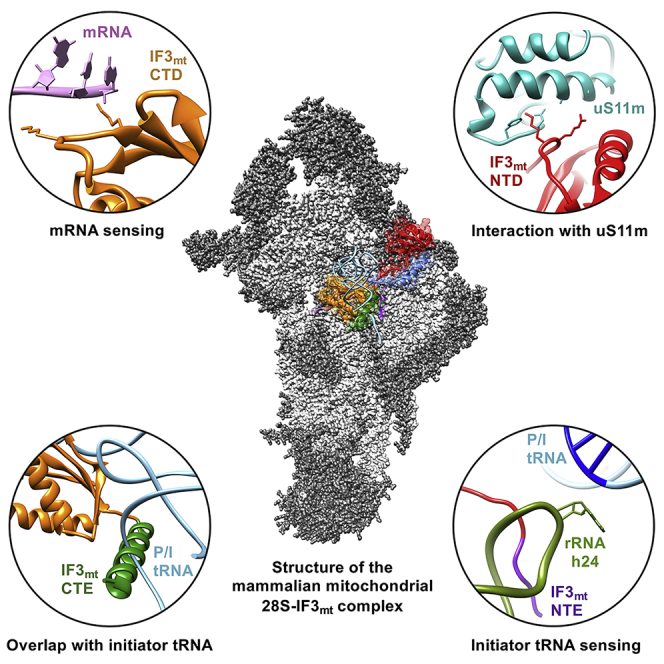
Summary
The human mitochondrial translational initiation factor 3 (IF3mt) carries mitochondrial-specific amino acid extensions at both its N and C termini (N- and C-terminal extensions [NTE and CTE, respectively]), when compared with its eubacterial counterpart. Here we present 3.3- to 3.5-Å-resolution cryoelectron microscopic structures of the mammalian 28S mitoribosomal subunit in complex with human IF3mt. Unique contacts observed between the 28S subunit and N-terminal domain of IF3mt explain its unusually high affinity for the 28S subunit, whereas the position of the mito-specific NTE suggests NTE's role in binding of initiator tRNA to the 28S subunit. The location of the C-terminal domain (CTD) clarifies its anti-association activity, whereas the orientation of the mito-specific CTE provides a mechanistic explanation for its role in destabilizing initiator tRNA in the absence of mRNA. Furthermore, our structure hints at a possible role of the CTD in recruiting leaderless mRNAs for translation initiation. Our findings highlight unique features of IF3mt in mitochondrial translation initiation.
Subject Areas: Biological Sciences, Structural Biology, Protein Structure Aspects
Graphical Abstract
Highlights
-
•
High-resolution cryo-EM study of the mammalian 28S mitoribosome-IF3mt complex
-
•
Interaction between the 28S and IF3mt's NTD explains NTD's unusual high affinity
-
•
Provides insights into role of IF3mt's N-terminal extension in initiator tRNA binding
-
•
Provides insights into roles of IF3mt's CTD and C-terminal extension in mRNA sensing
Biological Sciences; Structural Biology; Protein Structure Aspects
Introduction
Mitochondria are believed to have originated from the α-proteobacteria following an endosymbiotic event, in which the latter was engulfed by a primitive eukaryotic host cell (Gray et al., 2001). Although mitoribosomes have retained several structural and functional similarities from their bacterial ancestors, they acquired several novel features. One remarkable difference is the considerable truncation of the rRNAs in the mitoribosomes compared with their bacterial counterparts (Anderson et al., 1981). However, the loss of rRNA is partially compensated for by the acquisition of several mitochondrial-specific ribosomal proteins and extensions and/or insertions in the ribosomal proteins that have bacterial homologs (Amunts et al., 2015, Brown et al., 2014, Greber et al., 2014, Greber et al., 2015, Kaushal et al., 2014, Sharma et al., 2003). Although the overall steps of protein synthesis in mammalian mitochondria appear to be similar to those in bacteria, there are several key differences in terms of the interaction of mitoribosome with the mRNAs, the tRNAs, and the mitochondrial translational factors (Christian and Spremulli, 2012, Lightowlers et al., 2014, Sharma et al., 2013).
Translation initiation in bacteria has been widely explored through biochemical and structural studies, and the roles of all the three canonical bacterial initiation factors IF1, IF2, and IF3 are well established. All three IFs are essential for translation initiation in bacteria (Boelens and Gualerzi, 2002), whereas in mammalian mitochondria, homologs of only two bacterial factors, IF2mt and IF3mt, have been identified (Koc and Spremulli, 2002, Spencer and Spremulli, 2005). However, a mito-specific 37-amino-acid (aa)-long insertion domain in IF2mt has been proposed to mimic the function of bacterial IF1 during translation initiation in mammalian mitochondria (Gaur et al., 2008, Kummer et al., 2018, Yassin et al., 2011). In mimicking the function of IF1, the insertion domain in IF2mt sterically blocks the binding of initiator tRNA to the ribosomal A site (Yassin et al., 2011). IF2mt has been biochemically characterized in vitro, and it was shown to stimulate the binding of formyl-methionyl (fMet)-tRNAfMet to the small (28S) ribosomal subunit in the presence of mRNA (Ma and Spremulli, 1995, Spencer and Spremulli, 2005).
In bacteria, IF3 has been assigned multiple functions including discriminating elongator tRNAs from initiator tRNAs (Hartz et al., 1990, Hussain et al., 2016), promoting the dissociation of fMet-tRNAfMet at AUG codons on leaderless mRNAs (Tedin et al., 1999), and preventing the premature association of the 50S subunit with the 30S subunit until the formation of a proper pre-initiation complex (Hussain et al., 2016, Kaji et al., 2001, Zavialov et al., 2005). IF3 has also been implicated in the ribosome recycling process, although its exact role in this process has not been established. Bacterial IF3 has been reported to actively participate in the ribosomal subunit splitting event together with EF-G and RRF (Hirokawa et al., 2002, Kaji et al., 2001). At the same time, it has been suggested to act passively by removing the deacylated tRNA from the 30S subunit after the latter is dissociated from the 70S particle (Karimi et al., 1999, Zavialov et al., 2005). IF3mt stimulates the formation of initiation complexes at the 5′-terminal start codon on leaderless mRNAs that are specific to mitochondria (Christian and Spremulli, 2010). Unlike its bacterial homolog, which acts as an anti-association factor, IF3mt actively splits the 55S mitoribosomes by shifting the equilibrium between the 55S monosome and its two subunits (small 28S and large 39S) toward subunit dissociation (Koc and Spremulli, 2002). IF3mt has also been shown to dissociate the initiator fMet-tRNAfMet from the 28S subunit in the absence of mRNA (Bhargava and Spremulli, 2005, Haque and Spremulli, 2008). Using an E. coli translation system, IF3mt has been shown to promote the binding of initiator tRNAs containing the conserved 3GC base pairs in the anticodon stem (Ayyub et al., 2017).
Mammalian IF3mt has diverged considerably from its bacterial counterparts and retains only 20% to 25% sequence homology (Koc and Spremulli, 2002). Although the basic domain organization is very similar to bacterial IF3 comprising N- and C-terminal domains that are connected through a flexible linker, the mammalian mitochondrial factor has additional ∼30-aa-long extensions flanking both its termini (Bhargava and Spremulli, 2005, Haque et al., 2008, Koc and Spremulli, 2002). Deletions of these extensions have negative effects on the function of IF3mt during the initiation phase. Deletion of the C-terminal extension (CTE) renders the factor ineffective in destabilizing the incorrect initiation complexes, whereas deletion of the N-terminal extension (NTE) significantly increases the affinity of IF3mt for the 39S subunit (Bhargava and Spremulli, 2005, Haque and Spremulli, 2008). To further investigate the function of IF3mt in mitochondrial translational initiation and to understand the precise role of the mito-specific NTE and CTE, we have determined the cryoelectron microscopic (cryo-EM) structures of the human IF3mt bound to the bovine (Bos taurus) 28S subunit at 3.3 to 3.5 Å resolution.
Results and Discussion
Position of IF3mt on the 28S Mitoribosomal Subunit
We obtained a complex between the bovine 28S subunit and the human IF3mt by incubating the two components at 37°C for 5 min and obtained a 3D cryo-EM structure at 3.1 Å resolution (Figures S1C, S1D, and S2A). The map showed a strong density for readily recognizable body and platform domains of the subunit, whereas the density for the head domain was relatively weak, suggesting a conformational heterogeneity in the head domain. Moreover, a density corresponding to IF3mt could be readily identified in the map. To improve the density for the head domain, the dataset of 198,355 selected images was subjected to 3D classification, which yielded two major IF3mt-bound 28S mitoribosomal classes and a minor class that contained unusable images (Figure S1C). When the cryo-EM maps of the two IF3mt-bound classes were superimposed, the head regions of the two 28S subunit maps show relative rotation. The 28S-IF3mt complex that has conformation similar to that of the 28S subunit in the published human and porcine 55S mitochondrial ribosomal structures (Amunts et al., 2015, Greber et al., 2015) is referred to as Class I (resolution 3.5 Å) (Figures S2B and S3A; Table S1), and the class with conformational deviation is referred to as Class II (resolution 3.3 Å) (Figures S2C and S3B; Table S1). Between the two classes, rotation of the head domain along the 12S rRNA helix 28 (h28, base G524 served as the pivotal point of rotation) was estimated to be around 4.5° (Figure S3C). A similar type of head movement, generally known as head swiveling, has been reported previously in bacterial (Ratje et al., 2010, Schuwirth et al., 2005) and mammalian mitochondrial ribosomes (Amunts et al., 2015).
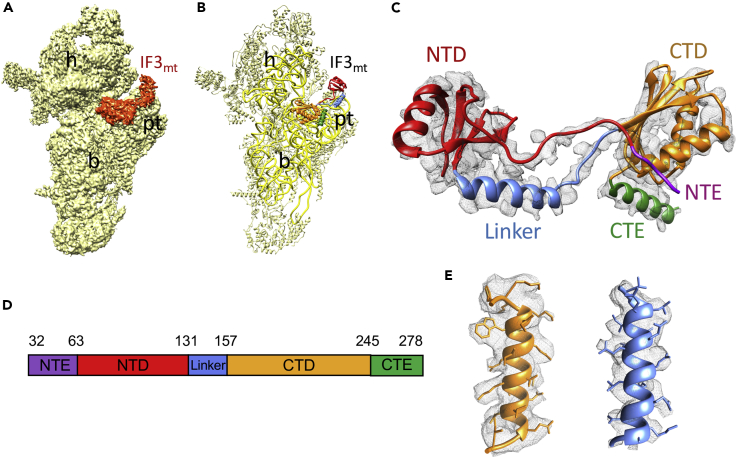
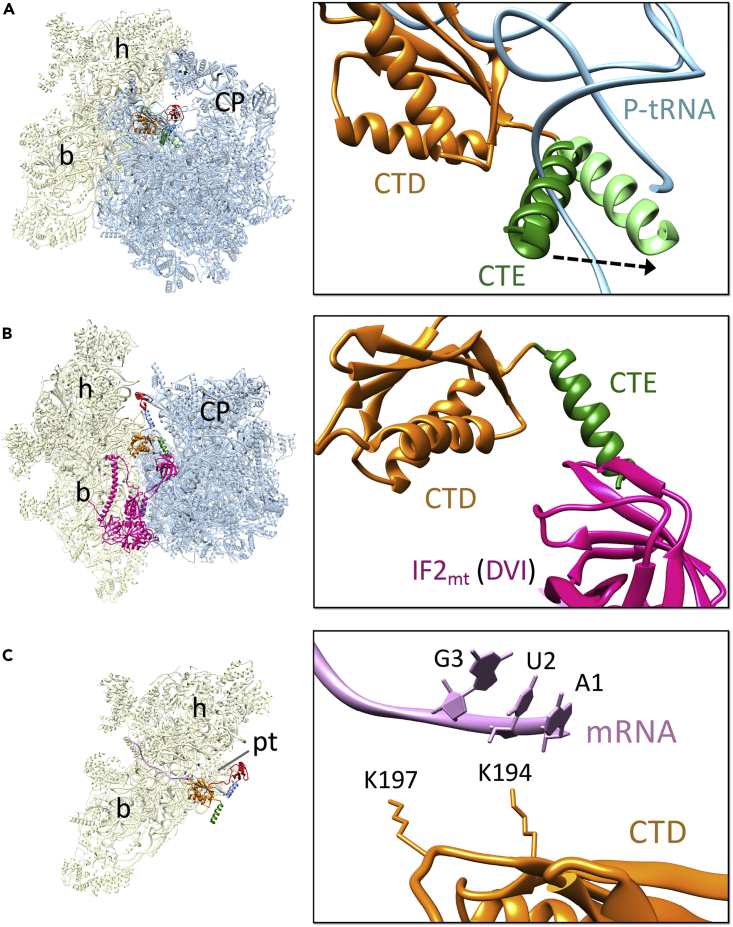
In both Class I and Class II maps a well-defined density, corresponding to two globular domains that were separated by a narrow helical region, could be readily identified as IF3mt (Figures 1A–1C and S4). However, despite substantial head movement, the position of IF3mt in the two classes remained almost unchanged (Figure S5A). Most of the interactions and conclusions presented in the upcoming sections of this article are based on Class II 28S-IF3mt complex, as it contained a better resolved IF3mt than in Class I and enabled near-atomic interpretations. Human IF3mt is composed of 278 aa where the first 31 residues constitute the mitochondrial target sequence (MTS) (Bhargava and Spremulli, 2005, Haque and Spremulli, 2008, Koc and Spremulli, 2002), a signal sequence necessary for the transport of IF3mt into the mitochondria. After the MTS is cleaved, the mature IF3mt is left with 247 aa that fold into two globular domains (Figures 1C and 1D). The overall binding position of IF3mt on the 28S subunit is consistent with the earlier published cryo-EM reconstructions of bacterial IF3 in complex with the 30S subunit (Hussain et al., 2016, Julian et al., 2011, Lopez-Alonso et al., 2017, McCutcheon et al., 1999) and site-directed hydroxyl radical probing experiments on 30S-IF3 complexes (Dallas and Noller, 2001, Fabbretti et al., 2007). The NTD is made up of a single α helix and four β sheet strands, whereas the CTD is composed of a four-stranded β sheet that is packed against two α helices (Figure 1C). The domains are connected through a helical linker region that confers conformational flexibility to both globular domains (Figure 1C). In the following sections, we describe the molecular interactions between the 28S subunit components and IF3mt, starting with the NTD, linker, CTD, and finally, mito-specific NTE and CTE.
Figure 1.
Cryo-EM Structure of the Mammalian Mitochondrial 28S-IF3mt Complex
(A) Three-dimensional cryo-EM map of the 28S mitoribosome-IF3mt complex, as viewed from the subunit's side that would face the large mitoribosomal subunit. The 28S subunit is shown in yellow, and the density corresponding to the IF3mt is shown in orange.
(B) Molecular interpretation of the cryo-EM map in (A). The 12S rRNA is displayed in bright yellow, whereas a lighter shade of yellow is used to mark the 28S ribosomal proteins (PDB ID: 3JD5). Landmarks of the 28S subunit: h, head; b, body; pt, platform.
(C) Completed model of IF3mt shown along with the corresponding cryo-EM density. Domains of IF3mt are color coded as in (D).
(D) Overall domain organization of the human IF3mt.
(E) Some of the segments of IF3mt with better resolved densities.
Interactions of the NTD
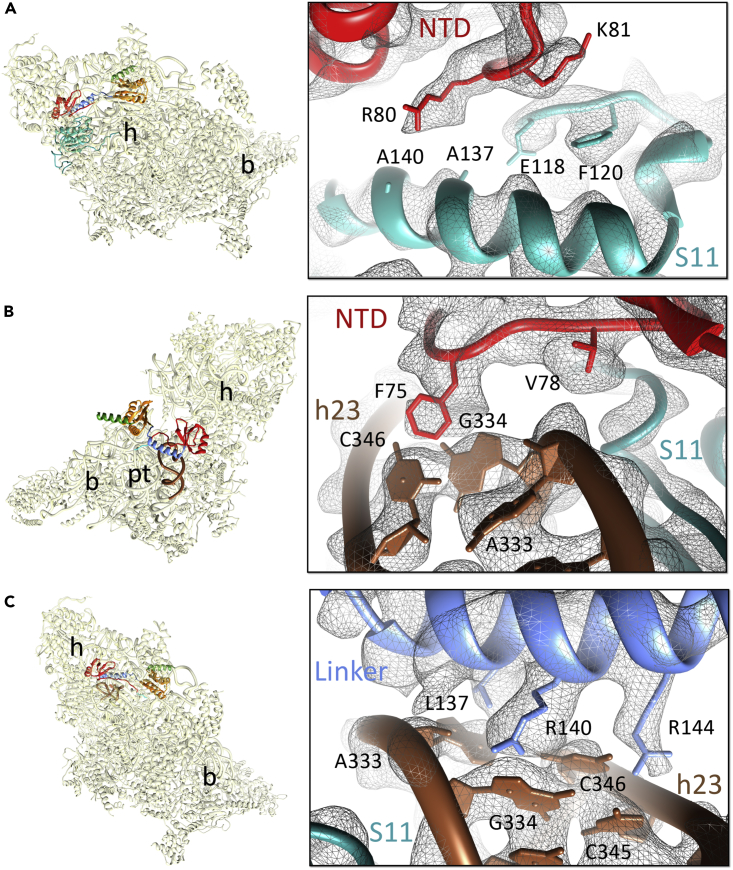
Of the two globular domains of IF3mt, its NTD binds closer to the cliff of the 28S subunit platform, in a position similar to that of the NTD of IF3 in the eubacterial complex. However, despite having a structural fold similar to that in a bacterial IF3 (Figure S5B), the orientation of the NTD in the mitoribosome structure is significantly different from that observed in the published bacterial 30S-IF3 complex structures (Figure S5C). None of the reported bacterial structures show any interaction between the NTD of IF3 and the 30S subunit (Hussain et al., 2016, Lopez-Alonso et al., 2017). In the mitochondrial complex, the NTD of IF3mt is oriented much closer to the 28S small subunit than the NTD of IF3 in any of the three 30S-IF3 complexes. This orientation allows the NTD to make multiple interactions with the adjacent ribosomal components such as mitoribosomal protein (MRP) uS11 and 12S rRNA helix 23 (h23). Despite the slightly lower resolution of the NTD (Figure S4), some of its bulky aa side chains could be traced. Arg80 in the NTD of IF3mt is found in close proximity to Ala137 and Ala140 of uS11m, whereas the adjacent Lys81 of the NTD is positioned close to Glu118 and Phe120 of the same MRP (Figure 2A). Val78 from the loop region of the NTD contacts the sugar moeity of h23 nucleotide (nt) A333, and the adjacent Phe75 is located in the vicinity of h23 nts G334 and C346 in an apparent stacking interaction (Figure 2B). It is highly likely that one of the major functions of the NTD of IF3mt is to provide additional anchoring points to enhance its affinity for the 28S subunit. This interpretation is consistent with the observation that the CTD in isolation can be readily displaced from the 28S subunit by the 39S subunit, whereas the full-length factor with the intact NTD binds much tighter and is difficult to displace from the 28S subunit (Haque and Spremulli, 2008). Furthermore, the NTD of IF3mt has been shown to bind independently to the 28S subunit with high affinity (Christian and Spremulli, 2012, Haque and Spremulli, 2008), whereas the NTD of its bacterial counterpart cannot interact with the 30S subunit without the presence of its CTD partner and the linker (Petrelli et al., 2001). Thus, unique interactions observed in our structure suggest a greater role for the IF3mt NTD in mitochondrial translation initiation than that of its counterpart in eubacterial translation.
Figure 2.
Interactions of the IF3mt NTD and the Linker Regions with the 28S Subunit
(A and B) (A) Interactions of the IF3mt NTD (red) with mitoribosomal protein S11 (cyan) and (B) 12S rRNA helix h23 (brown).
(C) Contacts between the IF3mt linker region (blue) and h23 of the 12S rRNA (brown). Thumbnails to the left represent overlaid positions of the ligands relative to the overall orientation to the 28S subunit (yellow). Landmarks on the thumbnail: h, head; b, body; pt, platform.
Interactions of the Linker
The linker region connecting the NTD and CTD is located on the rim of the 28S platform such that it runs over h23 of the 12S rRNA toward the P-site. Other than acting as a physical link between the NTD and CTD of IF3, the precise role of the linker region is not known in bacterial IF3 (Petrelli et al., 2001). However, in mitochondria the linker seems to enhance the affinities of both the NTD and the CTD for the 28S subunit (Haque and Spremulli, 2008). In bacteria, the only connection identified between the linker and the 30S ribosomal subunit is through a Tyr residue (Tyr75 according to E. coli numbering) that interacts with the C701 from h23 of the 16S rRNA (Hussain et al., 2016). Tyr75 of IF3 is highly conserved among bacteria and was suggested to play a crucial role in start codon discrimination and the selection of initiator tRNA (Hussain et al., 2016). Surprisingly, this important residue is not present in IF3mt. Instead, we find that two Arg residues (Arg140 and Arg144) and a Leu137 residue in IF3mt anchor the linker to the 28S platform by making multiple interactions with the 12S rRNA nts A333, G334, C345, and C346 of h23 (Figure 2C). Both the Arg residues are conserved in mammalian IF3mt (Figure S6A) and could have evolved to compensate for the loss of a single Tyr residue in eubacterial IF3 that was capable of providing stacking interactions.
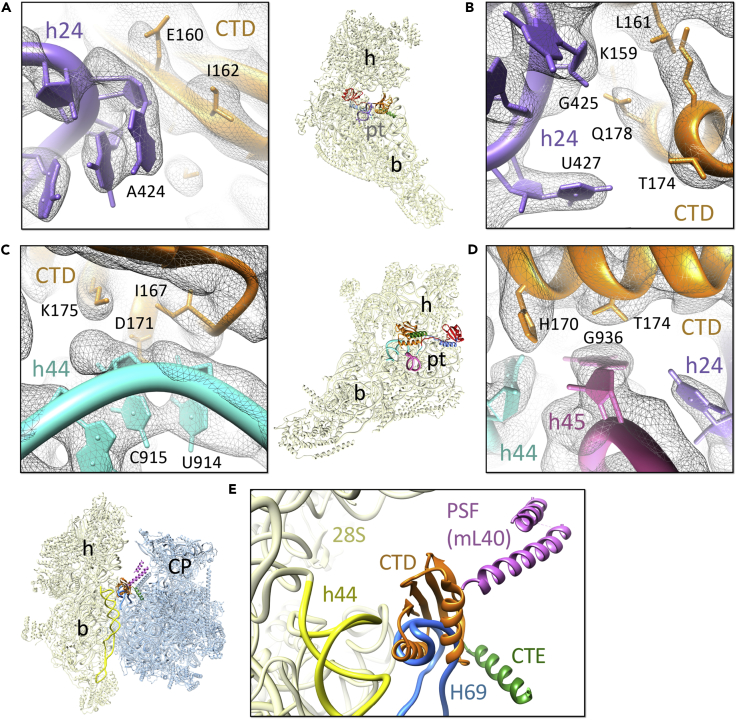
Interactions of the CTD
The CTD of IF3mt is positioned on the 28S platform in the vicinity of the P-site, and it interacts with several components of the 12S rRNA, including helices h24, h44, and h45. The rRNA nts from h24 provide the majority of the contact points for the CTD binding. Multiple nts from the apical loop of h24, including A424, G425, and U427 interact with the CTD residues Lys159, Glu160, Leu161, Ile162, Thr174, and Gln178 (Figures 3A and 3B). nts U914 and C915 from h44 provide additional contact points by interacting with Ile167 and Asp171, respectively, whereas G936 from h45 interacts with His170 and Thr174 of IF3mt CTD (Figures 3C and 3D). The majority of the aa residues that are involved in 28S-CTD interactions are conserved among human mitochondria and bacteria (Figure S6C) and are also reported in CTD-30S interactions in bacteria (Hussain et al., 2016). It should be noted that the sequence conservation between the human mitochondrial and bacterial IF3s is less than 25% (Koc and Spremulli, 2002). This kind of structural and functional conservation among two highly distinct kingdoms of life shows that although mitochondrial ribosomes diverged considerably from their bacterial ancestors, they retained key aa and nt residues to perform very specific functions.
Figure 3.
Interactions of the IF3mt CTD with the 28S Subunit and its Anti-association Property
(A–D) (A and B) The CTD (orange) provides major anchoring points for the stable binding of IF3mt by interacting with several 12S rRNA helices such as h24 (purple) and (C and D) h44 (cyan) and h45 (magenta). Thumbnails at the center represent overlaid positions of the ligands relative to the overall orientation to the 28S subunit (yellow).
(E) The CTD (orange) of IF3mt in its present conformation would prevent the association of 39S subunit with the 28S subunit by sterically blocking the formation of the central inter-subunit bridge, B2a, between h44 of the 28S subunit with H69 (blue), as well as docking of the mito-specific P-site finger (PSF, protein mL40, pink) from the large subunit. Landmarks on the thumbnail: CP, central protuberance of the large subunit; rest of the labels are the same as in Figure 2.
In the 28S-IF3mt complex, the position of the CTD is such that it would prevent the joining of the 39S subunit with the 28S subunit by directly interfering with the formation of two of the conserved inter-subunit bridges, B2a and B2b. The formation of bridge B2a requires binding with its partner helix 69 (H69) from the 16S rRNA in the 39S subunit (Figure 3E), Bridge B2b engages h24 from the 28S subunit and H67 from the 39S subunit (Greber et al., 2015, Kaushal et al., 2014). The location of the CTD would also prevent docking of the mito-specific P-site finger (protein mL40) from the 39S subunit during 55S formation (Figure 3E). The CTD of IF3mt thus safeguards the 28S subunit until a proper pre-initiation complex composed of IF2mt, mRNA, and initiator tRNA has been formed, a mechanism that has been well described for the bacterial system (Hussain et al., 2016).
Interactions of the Mito-Specific N- and C-Terminal Extensions
As described earlier, IF3mt possesses extensions on both its termini (Bhargava and Spremulli, 2005, Koc and Spremulli, 2002). The roles of these extensions during IF3mt-mediated mitochondrial initiation has been characterized through biochemical and mutational studies (Ayyub et al., 2017, Bhargava and Spremulli, 2005, Haque et al., 2008, Haque and Spremulli, 2008, Koc and Spremulli, 2002), but the function of these extensions at the molecular level was not known. Structure of the 28S-IF3mt complex presented here has helped us to directly visualize the interactions of both the NTE and CTE with the 28S subunit components and has enabled us to interpret their functional roles in mitochondrial translational initiation.
Role of the NTE
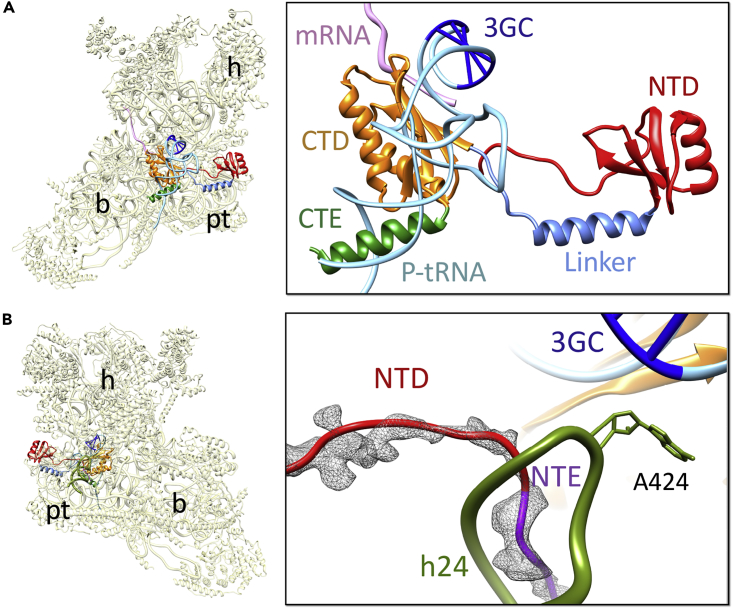
Using bacterial translation system, it was previously reported that IF3mt plays a role in maintaining translational fidelity by recognizing initiator tRNAs possessing the conserved 3GC base pairs in the anticodon stem and the NTD of IF3mt was proposed to be an important contributor to this function (Ayyub et al., 2017). However, structures of the bacterial 30S-IF3 complexes did not place the NTD anywhere close enough to the anticodon stem of the initiator tRNA to allow a direct interaction (Hussain et al., 2016, Lopez-Alonso et al., 2017) that would support a role of the NTD in discriminating against the non-initiator tRNAs. Similarly, in our 28S-IF3mt structure, the core of the NTD would not be positioned close to the 3GC pairs of the initiator tRNA, suggesting that the role of NTD of IF3mt in tRNA discrimination could only be indirect (Figure 4A). It was proposed that binding of IF3 induces conformational changes in the small subunit, allowing the conserved 16S rRNA bases G1338 and A1339 (according to E. coli numbering) to recognize the 3GC base pairs of the initiator tRNA through A-minor interactions (Dallas and Noller, 2001). Subsequent mutational studies (Lancaster and Noller, 2005) and crystallographic structures of the bacterial 70S ribosome complexes (Korostelev et al., 2006, Selmer et al., 2006), showing close proximity of G1338 and A1339 to the 3GC base pairs of the bound P-site tRNA, further cemented the importance of these rRNA residues in stabilizing the initiator tRNAs. Moreover, as the mammalian mitochondria carries a single tRNAMet with 3GC base pairs (Anderson et al., 1981) that is involved in both translation initiation and elongation steps, its 3GC base pair feature appears to be relevant during the initiation step in the selection of tRNAMet against the rest of the mitochondrial tRNAs.
Figure 4.
Relative Positions of NTD and NTE of IF3mt and Initiator tRNA
(A) The core of NTD (red) of IF3mt is situated away from the anticodon stem of the initiator tRNA (light blue) (M.R.S., R.K.K., N.K. Banavali, M.E.H., L.L.S., and R.K.A., unpublished data; Kummer et al., 2018) to interact with the conserved 3GC base pairs (dark blue) in the initiator tRNA. The mRNA (pink) is also shown. IF3mt domains are colored as in Figure 1.
(B) The NTD of IF3mt interacts with h24 (green) of 12S rRNA through its NTE (purple), whereas the h24 contacts the 3GC base pairs of the initiator tRNA with the help of a conserved adenine (A424). The cryo-EM density corresponding to the NTE (gray mesh) is also shown. Thumbnails to the left represent overlaid positions of the ligands relative to the overall orientation to the 28S subunit (yellow). Landmarks on the thumbnail: h, head; b, body; pt, platform.
Together with 3GC base pair interactions, additional interactions between the anticodon region and the ribosomal components have been proposed to be necessary for stable binding of the initiator tRNAs (Dallas and Noller, 2001). The highly conserved A790 located at the tip of h24 is known to interact with the anticodon region of the initiator tRNA and plays a crucial role during bacterial translation initiation (Fabbretti et al., 2007). In our Class II cryo-EM map, we found an additional low-resolution density close to the loop region of IF3mt-NTD that would extend from the core of the NTD toward the ribosomal P-site and could be readily assigned to the NTE of IF3mt (Figures 1C and 4B). The low resolution of the density did not allow us to model the NTE at the side chain level, but the backbone of the polypeptide chain could be traced. The NTE is positioned to make multiple interactions with h24 of the 12S rRNA thus aiding the high affinity of IF3mt for the small subunit. Most importantly, the NTE interacts with h24 carrying residue A424 (A790 in E. coli), and therefore, it is conceivable that the NTD through its NTE influences the binding of initiator tRNA in the P-site of the mitoribosome.
Role of the CTE
The high resolution of our structure allows us to model the CTE, except for the last 14 aa residues due to inconsistent local electron microscopic density. The CTE is composed of a single α helix, which is linked to the core CTD through a flexible loop region (Figures 1C and 5A). In the 28S-IF3mt complex, it is positioned away from the rim of the 28S platform and extends into what would be the inter-subunit side in a 55S mitoribosome complex. Interestingly, this is the only segment from the IF3mt that does not interact with any component of the 28S subunit. Although it does not have direct contacts with the 28S subunit, it is known to play an important role in mitochondrial translational initiation. When we superimposed our 28S-IF3mt structure on the structure of the mammalian mitochondrial 55S ribosome initiation complex comprising human IF2mt and initiator tRNA (M.R.S., R.K.K., N.K. Banavali, M.E.H., L.L.S., and R.K.A., unpublished date; Kummer et al., 2018), the CTE would prevent the binding of the initiator tRNA in the P-site position, as the α-helix of the CTE would be in steric clash with both the tRNA acceptor arm (Figure 5A) and domain VI of IF2mt (Figure 5B). Biochemical studies have suggested that IF3mt has the ability to destabilize the fMet-tRNA from the P-site of the 28S subunit in the absence of mRNA, but not if the 28S has a preloaded mRNA before the addition of fMet-tRNA (Bhargava and Spremulli, 2005, Christian and Spremulli, 2009). This unique function of IF3mt in sensing the precise order of events during mitochondrial initiation has not been reported in the bacterial system. Furthermore, the ability of IF3mt to dissociate the initiator tRNAs from the P-site in the absence of an mRNA is completely abolished if the CTE is removed from the factor (Bhargava and Spremulli, 2005, Christian and Spremulli, 2009). As our 28S-IF3mt complexes were devoid of an mRNA, the CTE is positioned in such a way that no tRNA binding would be allowed in the P-site. Without its CTE, the CTD alone would not be able to provide such steric hindrance to the P-site tRNA accommodation, explaining why this mito-specific mechanism is not observed in bacterial translation initiation, where IF3 lacks the CTE.
Figure 5.
Interactions between CTE and the Acceptor Arm of Initiator tRNA, and between Conserved Lysine Residues of CTD and mRNA
(A) The CTE (dark green) in its current position would directly block the binding of initiator tRNA (light blue) (M.R.S., R.K.K., N.K. Banavali, M.E.H., L.L.S., and R.K.A., unpublished data; Kummer et al., 2018) in the absence of mRNA. Detection of mRNA by the CTD (orange) allows tRNA binding in the P-site by changing the conformation of its CTE. The loop connecting the CTE with the rest of CTD permits conformational flexibility, enabling the CTE to adopt multiple positions (depicted in light green) away from the acceptor arm of the initiator tRNA.
(B) Absence of mRNA in our 28S-IF3mt complex positions the CTE in an orientation that will also occlude the binding of domain VI of IF2mt (dark pink).
(C) The CTD (orange) of IF3mt is positioned on the 28S subunit with two of its conserved lysines (K194 and K197) oriented toward the bound mRNA (light pink). Thumbnail to the left depicts an overall orientation of the 55S mitoribosome, with 28S (yellow) and 39S (blue) subunits, and overlaid positions of ligands. Landmarks on the thumbnail: 28S subunit: h, head; b, body; pt, platform; 39S subunit: CP, central protuberance.
Still the question remains how does IF3mt detect the presence of a bound mRNA and prevent the accommodation of the initiator tRNA in the absence of the mRNA. Our analysis of the ribosomal environment surrounding the CTD of IF3mt helps providing an explanation for the ability of IF3mt to detect the presence of the mRNA on the 28S subunit. Two Lys residues (Lys194 and 197) from the CTD of IF3mt are oriented toward the bound mRNA when our structure is superimposed to the mammalian mitochondrial 55S-IF2mt initiation complex (Figure 5C) (M.R.S., R.K.K., N.K. Banavali, M.E.H., L.L.S, and R.K.A., unpublished data; Kummer et al., 2018). Both these Lys residues along with Lys195 and Gly196 residues (KKGK motif) are conserved among mammals (Figure S6B). One of these lysines (Lys194) is placed very close to the nts A1 and U2 from the bound mRNA. It is worth mentioning that the KKGK motif is substituted by a highly conserved FRGR motif in the bacterial factor and the second Arg (Arg133 according to E. coli numbering) was found to interact with the +4 base of the bound mRNA (Hussain et al., 2016). This conserved Arg residue was proposed to function either to position the mRNA in place or to aid in codon-anticodon discrimination (Hussain et al., 2016). We cannot rule out the possibility that these Lys residues (K194 and K197) might function to hold the start codon in position thereby compensating for the absence of the SD sequence in mitochondrial mRNAs (Temperley et al., 2010). Considering their high level of conservation, particularly among mammals (Figure S7), we assume that the CTD of IF3mt would sense the binding of mRNA with the help of these Lys residues and communicate the signal to the CTE. Once the presence of the mRNA has been detected, the orientation of the CTE would be altered to allow the initiator tRNA to enter the P-site (Figure 5A). The sturdy α helix of the CTE is connected to the CTD through a loop that would confer a high level of flexibility to the CTE, and its strategic positioning in the inter-subunit side, without any interaction with the rest of the 28S-IF3mt complex, would allow its multi-directional movements. Based on these findings, we propose a plausible model (Figure 6) that could also be tested to help understand the mechanism of translation initiation in many other systems wherein IF3 possesses a CTE and mRNAs are leaderless. For example, in mycobacteria about one-third of the mRNAs are leaderless (Shell et al., 2015) and the IF3 in this organism possesses a CTE (Figure S6D).
Figure 6.
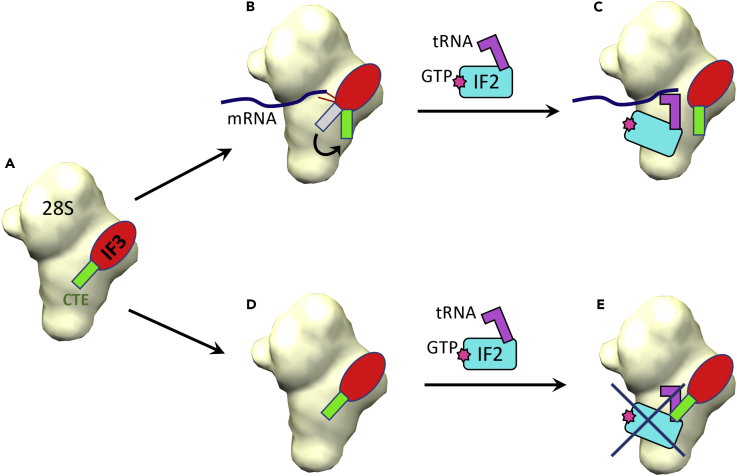
The Proposed Mechanism Depicting Roles of CTD and CTE in the Formation of Mammalian Translation Initiation Complex
(A) IF3mt binds to the 28S subunit, with its CTE in an extended position, as observed in our structure.
(B) If the mRNA is present, as probed by the conserved lysine residues in CTD of IF3mt, the position of CTE (green) changes to accommodate binding of the IF2mt·fMet-tRNAi·GTP complex.
(C) 28S initiation complex formed.
(D and E) (D) If the mRNA is absent, the CTE (green) remains in an extended position to (E) sterically block the binding of the IF2mt·fMet-tRNAi·GTP complex.
In conclusion, our study provides the high-resolution structure of the mitoribosome-bound human IF3mt and its mito-specific CTE and NTE, and their interactions with the mitoribosome, providing mechanistic explanations for several unique biochemical properties of IF3mt. Superimposition with the structure of the 55S·fMet-tRNAi·IF2mt complex allows us to interpret the role of the CTD and its mito-specific CTE in destabilizing the initiator tRNA in the absence of mRNA. Future structural studies capturing the simultaneous presence of mRNA and IF3mt and mRNA, IF3mt, and IF2mt on the 28S subunit should provide further insights into the mechanism of mitochondrial translation initiation.
Limitations of the Study
The model proposed in this study (Figure 6) is based on the structure of one of the functional states, i.e., binding of IF3mt to the 28S ribosomal subunit before mRNA and tRNA binding. Thus the proposed model is subject to test by solving the structure of additional states including mRNA, initiator tRNA, and IF2mt.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We acknowledge the use of the Wadsworth Center's and New York Structural Biology Center's (NYSBC's) EM facilities. NYSBC EM facilities are supported by grants from the Simons Foundation (349247), NYSTAR, the NIH (GM103310), and the Agouron Institute (F00316). We thank Kevin Elmore for help with purification of 28S subunits and IF3mt and ArDean Leith for help with computation. This work was supported by the NIH grants R01 GM61576 (to R.K.A.) and R01 GM32734 (to L.L.S.).
Author Contributions
R.K.A. and L.L.S. conceived this study. M.E.H. performed biochemical experiments. P.R. prepared cryo-EM samples and helped with data collection. R.K.K. and M.R.S. performed image processing. R.K.K. and R.K.A. analyzed the data and wrote the manuscript.
Declaration of Interests
Authors declare no competing financial interest in this work.
Published: February 22, 2019
Footnotes
Supplemental Information includes Transparent Methods, seven figures, and one table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.030.
Data and Software Availability
Both cryo-EM maps of the 28S subunit of the mammalian (Bos taurus) mitochondrial ribosome bound to human IF3 have been deposited in the Electron Microscopy and PDB DataBank (wwPDB.org) under accession codes EMD-9362 and PDB ID 6NF8 for Complex I and under accession codes EMD-9358 and PDB ID 6NEQ for Complex II.
Supplemental Information
References
- Amunts A., Brown A., Toots J., Scheres S.H.W., Ramakrishnan V. Ribosome. The structure of the human mitochondrial ribosome. Science. 2015;348:95–98. doi: 10.1126/science.aaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ayyub S.A., Aswathy S.L., Dobriyal D., Aluri S., Spremulli L.L., Varshney U. Fidelity of translation in the presence of mammalian mitochondrial initiation factor 3. Mitochondrion. 2017;39:1–8. doi: 10.1016/j.mito.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Bhargava K., Spremulli L.L. Role of the N- and C-terminal extensions on the activity of mammalian mitochondrial translational initiation factor 3. Nucleic Acids Res. 2005;33:7011–7018. doi: 10.1093/nar/gki1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens R., Gualerzi C.O. Structure and function of bacterial initiation factors. Curr. Protein Pept. Sci. 2002;3:107–119. doi: 10.2174/1389203023380765. [DOI] [PubMed] [Google Scholar]
- Brown A., Amunts A., Bai X.C., Sugimoto Y., Edwards P.C., Murshudov G., Scheres S.H.W., Ramakrishnan V. Structure of the large ribosomal subunit from human mitochondria. Science. 2014;346:718–722. doi: 10.1126/science.1258026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B.E., Spremulli L.L. Evidence for an active role of IF3mt in the initiation of translation in mammalian mitochondria. Biochemistry. 2009;48:3269–3278. doi: 10.1021/bi8023493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B.E., Spremulli L.L. Preferential selection of the 5'-terminal start codon on leaderless mRNAs by mammalian mitochondrial ribosomes. J. Biol. Chem. 2010;285:28379–28386. doi: 10.1074/jbc.M110.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B.E., Spremulli L.L. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta. 2012;1819:1035–1054. doi: 10.1016/j.bbagrm.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas A., Noller H.F. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol. Cell. 2001;8:855–864. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Fabbretti A., Pon C.L., Hennelly S.P., Hill W.E., Lodmell J.S., Gualerzi C.O. The real-time path of translation factor IF3 onto and off the ribosome. Mol. Cell. 2007;25:285–296. doi: 10.1016/j.molcel.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Gaur R., Grasso D., Datta P.P., Krishna P.D., Das G., Spencer A., Agrawal R.K., Spremulli L., Varshney U. A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. Mol. Cell. 2008;29:180–190. doi: 10.1016/j.molcel.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.W., Burger G., Lang B.F. The origin and early evolution of mitochondria. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-6-reviews1018. REVIEWS1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber B.J., Boehringer D., Leitner A., Bieri P., Voigts-Hoffmann F., Erzberger J.P., Leibundgut M., Aebersold R., Ban N. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature. 2014;505:515–519. doi: 10.1038/nature12890. [DOI] [PubMed] [Google Scholar]
- Greber B.J., Bieri P., Leibundgut M., Leitner A., Aebersold R., Boehringer D., Ban N. Ribosome. The complete structure of the 55S mammalian mitochondrial ribosome. Science. 2015;348:303–308. doi: 10.1126/science.aaa3872. [DOI] [PubMed] [Google Scholar]
- Haque M.E., Grasso D., Spremulli L.L. The interaction of mammalian mitochondrial translational initiation factor 3 with ribosomes: evolution of terminal extensions in IF3mt. Nucleic Acids Res. 2008;36:589–597. doi: 10.1093/nar/gkm1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.E., Spremulli L.L. Roles of the N- and C-terminal domains of mammalian mitochondrial initiation factor 3 in protein biosynthesis. J. Mol. Biol. 2008;384:929–940. doi: 10.1016/j.jmb.2008.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D., Binkley J., Hollingsworth T., Gold L. Domains of initiator tRNA and initiation codon crucial for initiator tRNA selection by Escherichia coli IF3. Genes Dev. 1990;4:1790–1800. doi: 10.1101/gad.4.10.1790. [DOI] [PubMed] [Google Scholar]
- Hirokawa G., Kiel M.C., Muto A., Selmer M., Raj V.S., Liljas A., Igarashi K., Kaji H., Kaji A. Post-termination complex disassembly by ribosome recycling factor, a functional tRNA mimic. EMBO J. 2002;21:2272–2281. doi: 10.1093/emboj/21.9.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T., Llacer J.L., Wimberly B.T., Kieft J.S., Ramakrishnan V. Large-scale movements of IF3 and tRNA during bacterial translation initiation. Cell. 2016;167:133–144.e13. doi: 10.1016/j.cell.2016.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian P., Milon P., Agirrezabala X., Lasso G., Gil D., Rodnina M.V., Valle M. The Cryo-EM structure of a complete 30S translation initiation complex from Escherichia coli. PLoS Biol. 2011;9:e1001095. doi: 10.1371/journal.pbio.1001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji A., Kiel M.C., Hirokawa G., Muto A.R., Inokuchi Y., Kaji H. The fourth step of protein synthesis: disassembly of the posttermination complex is catalyzed by elongation factor G and ribosome recycling factor, a near-perfect mimic of tRNA. Cold Spring Harb. Symp. Quant. Biol. 2001;66:515–529. doi: 10.1101/sqb.2001.66.515. [DOI] [PubMed] [Google Scholar]
- Karimi R., Pavlov M.Y., Buckingham R.H., Ehrenberg M. Novel roles for classical factors at the interface between translation termination and initiation. Mol. Cell. 1999;3:601–609. doi: 10.1016/s1097-2765(00)80353-6. [DOI] [PubMed] [Google Scholar]
- Kaushal P.S., Sharma M.R., Booth T.M., Haque E.M., Tung C.S., Sanbonmatsu K.Y., Spremulli L.L., Agrawal R.K. Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc. Natl. Acad. Sci. U S A. 2014;111:7284–7289. doi: 10.1073/pnas.1401657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc E.C., Spremulli L.L. Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs. J. Biol. Chem. 2002;277:35541–35549. doi: 10.1074/jbc.M202498200. [DOI] [PubMed] [Google Scholar]
- Korostelev A., Trakhanov S., Laurberg M., Noller H.F. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Kummer E., Leibundgut M., Rackham O., Lee R.G., Boehringer D., Filipovska A., Ban N. Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. Nature. 2018;560:263–267. doi: 10.1038/s41586-018-0373-y. [DOI] [PubMed] [Google Scholar]
- Lancaster L., Noller H.F. Involvement of 16S rRNA nucleotides G1338 and A1339 in discrimination of initiator tRNA. Mol. Cell. 2005;20:623–632. doi: 10.1016/j.molcel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lightowlers R.N., Rozanska A., Chrzanowska-Lightowlers Z.M. Mitochondrial protein synthesis: figuring the fundamentals, complexities and complications, of mammalian mitochondrial translation. FEBS Lett. 2014;588:2496–2503. doi: 10.1016/j.febslet.2014.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alonso J.P., Fabbretti A., Kaminishi T., Iturrioz I., Brandi L., Gil-Carton D., Gualerzi C.O., Fucini P., Connell S.R. Structure of a 30S pre-initiation complex stalled by GE81112 reveals structural parallels in bacterial and eukaryotic protein synthesis initiation pathways. Nucleic Acids Res. 2017;45:2179–2187. doi: 10.1093/nar/gkw1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Spremulli L.L. Cloning and sequence analysis of the human mitochondrial translational initiation factor 2 cDNA. J. Biol. Chem. 1995;270:1859–1865. doi: 10.1074/jbc.270.4.1859. [DOI] [PubMed] [Google Scholar]
- McCutcheon J.P., Agrawal R.K., Philips S.M., Grassucci R.A., Gerchman S.E., Clemons W.M., Jr., Ramakrishnan V., Frank J. Location of translational initiation factor IF3 on the small ribosomal subunit. Proc. Natl. Acad. Sci. U S A. 1999;96:4301–4306. doi: 10.1073/pnas.96.8.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli D., LaTeana A., Garofalo C., Spurio R., Pon C.L., Gualerzi C.O. Translation initiation factor IF3: two domains, five functions, one mechanism? EMBO J. 2001;20:4560–4569. doi: 10.1093/emboj/20.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratje A.H., Loerke J., Mikolajka A., Brunner M., Hildebrand P.W., Starosta A.L., Donhofer A., Connell S.R., Fucini P., Mielke T. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature. 2010;468:713–716. doi: 10.1038/nature09547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuwirth B.S., Borovinskaya M.A., Hau C.W., Zhang W., Vila-Sanjurjo A., Holton J.M., Cate J.H. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- Selmer M., Dunham C.M., Murphy F.V.t., Weixlbaumer A., Petry S., Kelley A.C., Weir J.R., Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Sharma M.R., Koc E.C., Datta P.P., Booth T.M., Spremulli L.L., Agrawal R.K. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- Sharma M.R., Kaushal P.S., Gupta M., Banavali N.K., Agrawal R.K. Insights into structural basis of mammalian mitochondrial translation. In: Duchene A.-M., editor. Translation in Mitochondria and Other Organelles. Springer; 2013. pp. 1–28. [Google Scholar]
- Shell S.S., Wang J., Lapierre P., Mir M., Chase M.R., Pyle M.M., Gawande R., Ahmad R., Sarracino D.A., Ioerger T.R. Leaderless transcripts and small proteins are common features of the mycobacterial translational landscape. PLoS Genet. 2015;11:e1005641. doi: 10.1371/journal.pgen.1005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A.C., Spremulli L.L. The interaction of mitochondrial translational initiation factor 2 with the small ribosomal subunit. Biochim. Biophys. Acta. 2005;1750:69–81. doi: 10.1016/j.bbapap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Tedin K., Moll I., Grill S., Resch A., Graschopf A., Gualerzi C.O., Blasi U. Translation initiation factor 3 antagonizes authentic start codon selection on leaderless mRNAs. Mol. Microbiol. 1999;31:67–77. doi: 10.1046/j.1365-2958.1999.01147.x. [DOI] [PubMed] [Google Scholar]
- Temperley R.J., Wydro M., Lightowlers R.N., Chrzanowska-Lightowlers Z.M. Human mitochondrial mRNAs–like members of all families, similar but different. Biochim. Biophys. Acta. 2010;1797:1081–1085. doi: 10.1016/j.bbabio.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin A.S., Haque M.E., Datta P.P., Elmore K., Banavali N.K., Spremulli L.L., Agrawal R.K. Insertion domain within mammalian mitochondrial translation initiation factor 2 serves the role of eubacterial initiation factor 1. Proc. Natl. Acad. Sci. U S A. 2011;108:3918–3923. doi: 10.1073/pnas.1017425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavialov A.V., Hauryliuk V.V., Ehrenberg M. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol. Cell. 2005;18:675–686. doi: 10.1016/j.molcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.