Abstract
Laser refractive surgery is one of the most performed surgical procedures in the world. Although regarded safe and efficient, it has side effects. All of the laser based refractive surgical procedures invoke corneal nerve injury to some degree. The impact of this denervation can range from mild discomfort to neurotrophic corneas. Currently, three techniques are widely used for laser vision correction: small incision lenticule extraction, laser-assisted keratomileusis in situ and photorefractive keratotomy. Each of these techniques affects corneal innervation differently and has a different pattern of nerve regeneration. The purpose of this review is to summarize the different underlying mechanisms for corneal nerve injury and compare the different patterns of corneal reinnervation.
Keywords: photorefractive keratotomy, small incision manual lenticule extraction, laser-assisted keratomileusis in situ, refractive surgery, in vivo confocal microscopy, corneal sensation, corneal nerve
Introduction
About 50–60% of the global adult population suffers from a refractive error (Solomon et al., 2009; Williams et al., 2015). A recent study estimated that there were 1406 million people with myopia in 2000 and this number will reach 4578 million (49.8% of the world population) by 2050 (Holden et al., 2016).
Laser refractive surgery is a therapeutic procedure that aims to replace glass prescription, by surgically removing corneal stromal tissue to change the shape and power of the cornea (Seiler and Wollensak, 1986). Recent reports have endorsed laser refractive surgery as a relatively safe and effective treatment (Wen et al., 2017) with high satisfaction indexes (95–98%) (Sandoval et al., 2016), especially if compared to other cosmetic procedures (Frost et al., 2000; Sommer et al., 2003; Booth et al., 2004; Honigman et al., 2004; Schwitzer et al., 2015; Sandoval et al., 2016). One example of its safety is that even the US military forces have approved the technology for the use in soldiers, navy pilots and National Aeronautics and Space Administration (NASA) astronaut candidates (Stanley et al., 2008).
It is the most common performed surgical procedure in the World, more than 16 millions surgeries have been performed since its introduction in the ophthalmology practice (Solomon et al., 2009). However, its popularity does not mean that every case is successful. The most common side effect of any laser procedure is the transection of the corneal nerve plexus. This may lead to dry eye disease, neurotrophic epitheliopathy/keratopathy and loss of corneal sensitivity (Dohlman et al., 2016). In this review, we will discuss the different aspects of post-operative corneal reinnervation and sensation among the currently available refractive laser surgery techniques.
Search Strategy and Selection Criteria
The following databases are used: Google Scholar, PubMed, PubMed Central, Research Gate. Common keywords were corneal refractive surgery; myopia; laser assisted in situ keratomileusis (LASIK); photorrefractive keratectomy (PRK); small incision lenticule extraction (SMILE); corneal nerves; hyposthesia; neurotrophic cornea; corneal reinnervation; subbasal nerve plexus; subepithelial nerve plexus. We restricted searches to studies in English, including reviews, in vitro studies, studies conducted on humans and animals, and metanalyses, which published from 1950 to April 2018. The search strategy and selection criteria are shown in Table 1.
Table 1.
Search strategy and selection criteria
| Database | Google Scholar, PubMed, PubMed Central, Research Gate |
| Date | 1950 - April 2018 |
| Eligibility criteria | Reviews, in vitro studies, Studies conducted on humans and animals, metanalyses, and published in English |
| Keywords/keyterms | corneal refractive surgery; myopia; laser assisted in situ keratomileusis (LASIK); photorrefractive keratectomy (PRK); small incision lenticule extraction (SMILE); corneal nerves; hyposthenia; neurotrophic cornea; corneal reinnervation; subbasal nerve plexus; subepithelial nerve plexus |
Corneal Innervation: Anatomy and Function
The cornea is around 520–560 μm thick (Dimasi et al., 2010) (Figure 1) and with over 16,000 nerve terminations/mm3 (Guthoff et al., 2005), it is the most densely innervated tissue in the human body, with sympathetic and parasympathetic nerve fibers running through it (Marfurt et al., 1989). The high corneal nerve density within the epithelium sets a very low threshold for the detection of external stimuli, hence allowing a prompt and prominent defensive reflex, crucial in the protection of the ocular surface (Oliveira-Soto and Efron, 2001; Guthoff et al., 2005; Cruzat et al., 2010).
Figure 1.
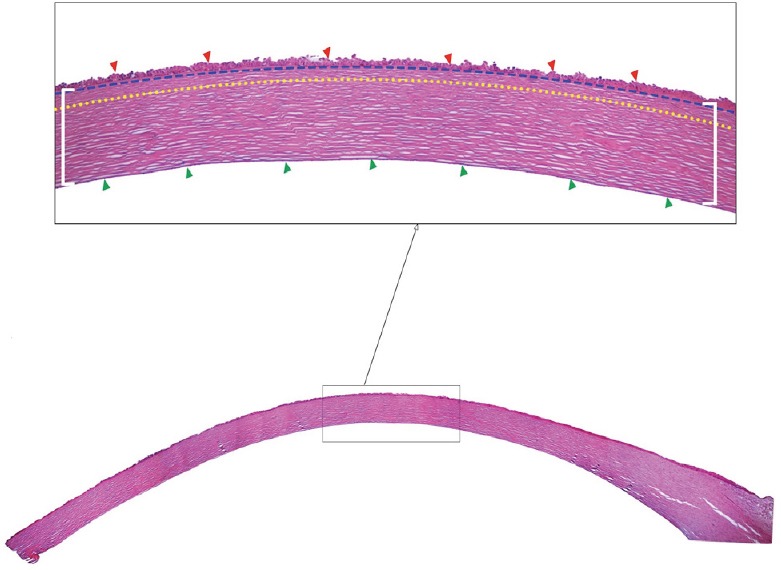
Histological crossection of a human cornea (hematoxylin-eosin staining).
Montage of a whole cornea and an insert of the corneal layers (4× magnification). The red arrowheads correspond to the epithelial layer; the corneal stroma is comprised between the white brackets; and the green arrowheads delineate the endothelial layer. The Bowman layer is delimited by the blue dashed and yellow dotted lines. The image is provided by Singapore Eye Research Institute Image Library.
Corneal nerves are separated into terminal endings/receptors and there are three distinct plexuses according to depth, orientation and size. They are described below as follows.
Terminal endings and receptors
Corneal nerve ending terminals present as 3 different types of receptors: 10–20% are mechanoreceptors (sensitive to mechanical forces), 10% are thermal receptors (sensitive to cooling), and the rest are polymodal receptors (sensitive to a variety of stimuli, e.g., heat, chemical and endogenous molecules). Polymodal and mechanoreceptors will elicit a sensation of ocular surface discomfort and pain (Belmonte, 2007).
Sub-basal nerve plexus
After breaking through bowman layer, the corneal nerves subdivide further onto smaller branches and these fibers run in between epithelial cells and will result in the free nerve endings with receptors at the most superficial epithelial layer (or wing cell layers) (Muller et al., 2003; Eguchi et al., 2017). They consist of both beaded and straight fibers, the former are found at the periphery of the bundle and correspond to axonal efferent and sensory terminals (Patel and McGhee, 2009).
Sub-bowman nerve plexus
The stromal bundles subdivide and turn 90º perpendicular to the corneal epithelium and travel between the anterior stroma and Bowman layer. This plexus is distributed sparsely and patchy with most of the network located in the mid-peripheral cornea (Muller et al., 2003; Benitez-Del-Castillo et al., 2007; Patel and McGhee, 2009) at a depth between 70–160 µm (Schmoll et al., 2012; Lopez de la Fuente et al., 2016). These nerves will penetrate the Bowman layer, again they turn 90º and are situated between the basal epithelium and anteriorly to the Bowman layer (Al-Aqaba et al., 2010) (Figure 2).
Figure 2.
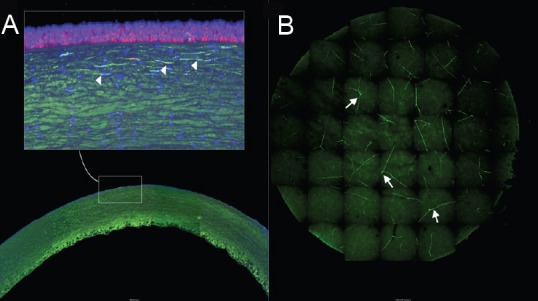
Corneal nerve distribution.
Confocal microscopy montage of a whole cornea mount stained with DAPI (blue); phalloidin (red) and TuJ-1 (green). The nerve distribution is shown in two different perspectives. (A) Crossection of the whole cornea 5× magnification, the white square shows a 20× magnification, the white arrowheads show intense green signal – which are the stromal and sub-basal nerves running parallel to collagen fibers. (B) Flat-mount confocal 3D image of a SMILE lenticule; images from the top to bottom of the lenticule were taken 5 µm apart and stacked into a single frame; the white arrows indicate the disposition of corneal nerves. SMILE: Small incision manual lenticule extraction; DAPI: 4’,6-diamidino-2-phenylindole. The image is provided by Singapore Eye Research Institute Image Library.
Stromal nerves
Corneal nerve bundles originate from the ophthalmic branch of the trigeminal nerve and in the conjunctiva they form a plexus and enter the peripheral limbus in a radial centripetal fashion, parallel to the collagen fibers. They lose their myelin sheath 1 mm after entering into the cornea, at the level of the anterior/mid stroma. These are the largest and thickest nerves in the cornea, and they are found at a lower density, when compared to the more superficial corneal nerve plexuses (Oliveira-Soto and Efron, 2001; Muller et al., 2003; Patel and McGhee, 2009).
Neural pathway
Sensation from the ocular surface is then transmitted to interpretative regions of the brain. Primary sensory nerves travel through the trigeminal ganglion sensory root to the pons and trigeminal nuclei, where they synapse with second-order neurons at the dorsal horn of the spinal cord and head to the thalamus via spinothalamic pathways. Third-order neurons leave the thalamus to the cortex, where impulses will be interpreted as pain, dryness, irritation or cooling (Rosenthal et al., 2009).
Corneal nerves and ocular surface homeostasis
Besides signaling pain and other sensations, corneal nerves also play a role in maintaining the ocular surface homeostasis. Upon irritant stimuli, they release trophic factors, such as neuropeptides, that help to preserve the corneal integrity upon inflammation (Tervo et al., 1982; Belmonte, 2007) and the polymodal nociceptors are activated evoking defensive reflexes such as tearing and blinking (Beuerman and Schimmelpfennig, 1980; Acosta et al., 2004).
Laser Refractive Surgery
Background
The rationale behind refractive surgery is changing the power of the cornea to correct the refractive errors. First reports of refractive surgery with a laser date back to 1980’s in both animals (Trokel et al., 1983) and humans (Seiler, 1990).
Subsequently, both Pallikaris and Buratto (Buratto et al., 1992; Pallikaris and Siganos, 1994), described a technique that combined lamellar corneal surgery with a microkeratome (Barraquer, 1967) and an excimer laser ablation, i.e., laser-assisted keratomileusis in situ (LASIK). More recently, the femtosecond laser has been adopted to replace the microkeratome blade.
Lasers mechanisms
Excimer laser
The excimer laser is an argon fluoride solid-state laser of a 193 nm wavelength that allows precise corneal tissue excision through a photochemical tissue-laser interaction. It promotes an ablative photodecomposition that directly breaks organic molecular bonds (including collagen, epithelium, keratocytes and nerve bundles) producing a gas under high pressure without tissue heating, hence it does not induce necrosis on surrounding or underlying tissue (Trokel et al., 1983).
Femtosecond laser
The femtosecond laser produces ultrashort pulses at a very high intensity, with a wavelength of 1053 nm, achieving a very precise cutting effect by overcoming the plasma formation threshold, leading to an optical breakdown in ocular tissue structures (Davis et al., 1991). This process is called photodisruption.
Surgical techniques that affect corneal nerves
The endpoint of refractive surgery is to change the shape of the cornea, which will reduce or increase its refractive power, hence correcting the refractive error. Three techniques are commonly used to this purpose: photo-refractive keratotomy (PRK), LASIK, and small incision lenticule extraction (SMILE) (Figure 3).
Figure 3.
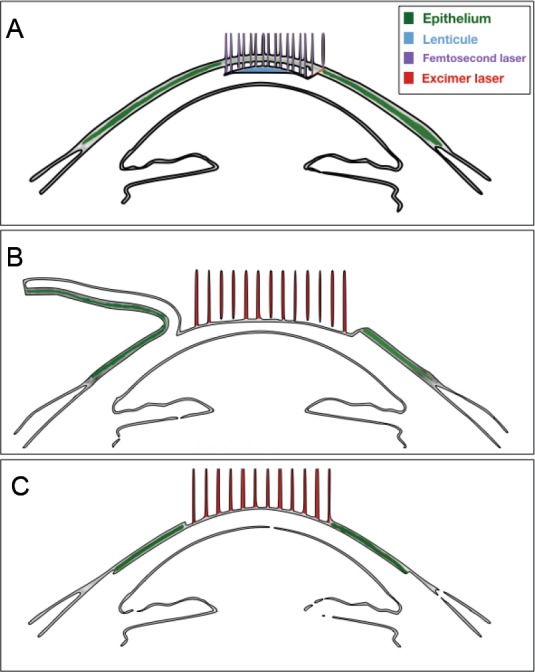
Schematics of the different refractive surgical procedures.
(A) Small incision lenticule extraction; (B) laser-assisted keratomileusis in situ; (C) photorrefractive keratectomy.
PRK
In PRK, corneal de-epithelialization is performed mechanically or chemically (Carones et al., 1999), and stromal tissue is removed solely with an excimer laser photoablation to the corneal epithelial basement membrane and anterior stroma (Mohan et al., 2003). With this technique, there is no transection of deep stromal nerves. In most cases, the laser treatment only affects the sub-basal and more superficial sub-bowman nerves. However, it is a painful procedure, since after the ablation the remaining nerve endings are exposed at the corneal surface until the epithelium grows over the surgical wound (2–10 days) (Mohan et al., 2003).
LASIK
LASIK consists of using a microkeratome or laser (Yesilirmak et al., 2016) to create a flap in the anterior stroma between 110–160 μm in depth, just below the epithelial basement membrane. The flap is lifted and photoablation is performed to the stromal portion of the cornea. There is minimal epithelial breakdown, and basal membrane damage, because they are restricted to the side-cut. The post-operative pain sensation is minimal because there is no exposure of nerve endings (Mohan et al., 2003). However, the lamellar ablation severes stromal nerve and the rim cut transects the sub-bowman nerves, de-innervating all the incision area, only preserving nerves coming from the hinge (Latvala et al., 1996b).
SMILE
In SMILE, corneal tissue is disrupted by a femtosecond laser, in such a manner to create a lenticule of the desired refractive power, which is then removed manually through a small incision (Sekundo et al., 2011). This approach aimed to be less invasive than LASIK, as the incision is smaller and the excised lenticule can be cut in a deeper plane. In theory, this approach should spare nerves that are more superficial.
Surgical planning and effect on corneal innervation
The volume of tissue removal/ablation will be dependent on the type of refractive error (Gatinel et al., 2002a, b) and this will have a direct effect on the post-operative corneal nerve density (Campos et al., 1992; Tervo et al., 1994). The different amount of denervation found amongst treatments is due to the specific surgical planning required for each of them and its relationship with the uneven distribution of the corneal nerve density, which is higher in the periphery and lower at the center (Latvala et al., 1996a; Tervo and Moilanen, 2003).
For instance, hyperopic patients will undergo subtraction of peripheral tissue in such a way to obtain a steeper cornea. Hence, this ablation profile will affect more nerves, since corneal nerve density is higher at the site where the ablation is most intense. Whereas in myopic surgical profiles, the tissue removal will be focused mostly on the central part, to obtain a flatter cornea (Azar and Primack, 2000). The zone of treatment in myopic surgery harms less nerves than hyperopic treatments, because the corneal nerve density in the center of the cornea is relatively less than in the periphery (Latvala et al., 1996a).
The amount of corneal nerve loss will also depend on other factors, such as: diameter of lenticule (SMILE); ablation zone (PRK/LASIK) (Latvala et al., 1996b); flap size (Feng et al., 2013); and degree of the refractive error (Campos et al., 1992). For LASIK and PRK, the reduction in corneal nerve density will be directly proportional to the diameter of ablation/flap and to the degree of correction intended (Latvala et al., 1996a; Tervo and Moilanen, 2003).
Consequences of Nerve Transection and Reinnervation after Refractive Surgery
The two major consequences of the corneal nerve density reduction with refractive surgery are neuropathic keratopathy and dry eye disease. Symptoms of dry eye disease are common after all laser refractive surgery and the most frequent symptoms are irritation, burning, foreign body sensation and epiphora, along with fluctuations in vision (Dohlman et al., 2016). More severe consequences are hyposthesia, delay in corneal epithelialization, neutrophic ulcers and chronic inflammation due to dry eye (Chao et al., 2014).
Clinical studies rely on in vivo confocal microscopy (IVCM) and esthesiometers to assess the reinnervation (Tables 2 and 3, and Figure 4) (Lee et al., 2002, 2005; Calvillo et al., 2004; Erie et al., 2005; Darwish et al., 2007; Patel and McGhee, 2009; Li et al., 2013; Vestergaard et al., 2013; Mohamed-Noriega et al., 2014; Agca et al., 2015; Chao et al., 2015; Ishii et al., 2015; Liu et al., 2015) and recovery of corneal sensation (Table 4 and Figure 5) (Ishikawa et al., 1994; Perez-Santonja et al., 1999; Benitez-del-Castillo et al., 2001; Bragheeth and Dua, 2005; Lee et al., 2005; Nejima et al., 2005; Darwish et al., 2007; Patel and McGhee, 2009; Li et al., 2013; Vestergaard et al., 2013; Wei and Wang, 2013; Kung et al., 2014; Chao et al., 2015; He et al., 2015b; Xia et al., 2016), respectively.
Table 2.
Early corneal re-innervation (%)
| n | Pre-operative | 1 month | 3 months | 6 months | |
|---|---|---|---|---|---|
| PRK | 90 | 100 | 19±19 | 28±24 | 50±17 |
| LASIK | 172 | 100 | 11±17 | 24±21 | 27±21 |
| SMILE | 156 | 100 | 55±34 | 53±41 | 58±35 |
Data are expresed as the mean ± SD. PRK: Photorefractive keratotomy; LASIK: laser-assisted keratomileusis in situ; SMILE: small incision manual lenticule extraction.
Table 3.
Late corneal re-innervation (%)
| n | 1 year | 2 years | 3 years | 5 years | |
|---|---|---|---|---|---|
| PRK | 90 | 40.73±19.00 | 91.98±26.00 | 93.69±23.00 | 86.99±45.00 |
| LASIK | 172 | 44.00±16.00 | 58.00±10.00 | 57.00±24.00 | 79.00 |
Data are expresed as the mean ± SD. PRK: Photorefractive keratotomy; LASIK: laser-assisted keratomileusis in situ.
Figure 4.
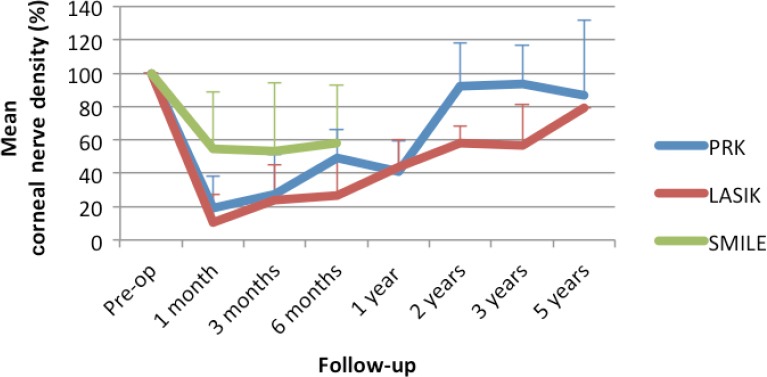
Corneal re-innervation.
The chart displays the mean recovery of corneal nerve density over time compared to baseline. The colored lines represent the different procedures: blue for PRK; red for LASIK; and green for SMILE. The error bars represent the standard deviation. PRK: Photorefractive keratotomy; LASIK: laser-assisted keratomileusis in situ; SMILE: small incision manual lenticule extraction.
Table 4.
Corneal sensitivity recovery (%)
| n | Pre- operative | 1 week | 1 month | 3 months | 6 months | 1 year | |
|---|---|---|---|---|---|---|---|
| PRK | 118 | 100 | 81±39 | 91±38 | 98±26 | 100±12 | 100 |
| LASIK | 792 | 100 | 25±24 | 46±32 | 56±29 | 77±16 | 95±10 |
| SMILE | 202 | 100 | 76±30 | 79±29 | 87±24 | 86±19 | – |
Data are expresed as the mean ± SD. PRK: Photorefractive keratotomy; LASIK: laser-assisted keratomileusis in situ; SMILE: small incision manual lenticule extraction.
Figure 5.
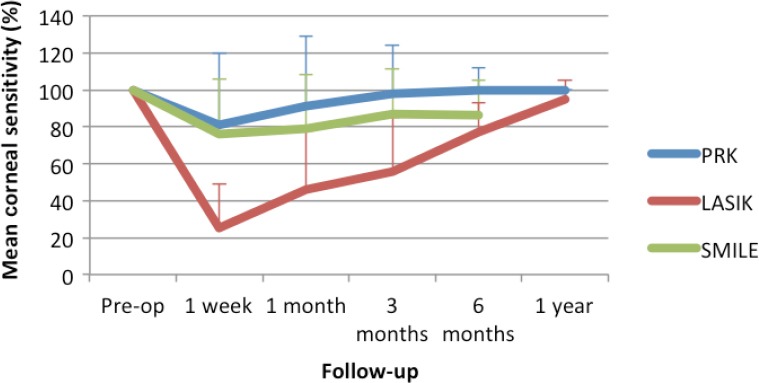
Corneal sensitivity recovery.
The chart displays the mean recovery of corneal sensitivity over time compared to baseline. The colored lines represent the different procedures: blue for PRK; red for LASIK; and green for SMILE. The error bars represent the standard deviation. PRK: Photorefractive keratotomy; LASIK: laser-assisted keratomileusis in situ; SMILE: small incision manual lenticule extraction.
IVCM acquires several images at a fixed depth, layer by layer, by focusing into a single corneal plane with significant resolution to allow the identification of corneal nerves (Sonigo et al., 2006). The Cochet-bonnet and Belmonte esthesiometers can determine the threshold of corneal reflexes to different stimuli (Belmonte et al., 1999).
PRK reinnervation
During PRK, only the epithelium and most anterior stroma are removed, this allows new neurites to arise from the severed nerve endings directly into the epithelial-stromal interface. New nerve fibers start to emerge from the ablation area as early as 1–7 days after PRK and about 50% of the reinnervation is complete between 3 to 6 months (Erie et al., 2005; Lee et al., 2005; Darwish et al., 2007), but morphological and functional changes may still be present after 12 months (Tervo and Moilanen, 2003).
IVCM showed the sub-basal nerves reappearing around seven days and there are histological studies demonstrating different morphological features of newly regenerated nerves, such as sprouting of sub-basal nerves and irregular branching/coiling (Tervo et al., 1994). Recent studies have shown that mean sub-basal density of corneal nerves regenerates gradually, but it remains reduced by 59% from baseline over a year after surgery and only returns to preoperative density after 2 years (Erie et al., 2005).
The mean sensitivity after PRK returns to 75% of baseline values within 6 postoperative weeks and in 3 months patients may recover between 85–95% of the sensitivity (Perez-Santonja et al., 1999; Lee et al., 2005; Nejima et al., 2005; Darwish et al., 2007). There is a direct correlation between the degree and speed of sensitivity recovery with the amount of laser correction (Campos et al., 1992).
LASIK reinnervation
The stromal and sub-basal nerves are both severed during the flap creation, with the exception of those located at the flap hinge; subsequently the excimer laser will ablate the underlying stromal nerve plexus. Nerve regeneration starts in the peripheral margins of the flap and move slowly in a centripetal direction to the center of the cornea, and these fibers originate mainly from the flap hinge (Latvala et al., 1996a; Feng et al., 2013).
IVCM shows that intense denervation can last up to three months (Calvillo et al., 2004; Erie et al., 2005; Lee et al., 2005; Darwish et al., 2007; Li et al., 2013; Vestergaard et al., 2013; Mohamed-Noriega et al., 2014; Agca et al., 2015; Chao et al., 2015). Less than 10% of the original sub-basal nerves remains in the operated cornea (Lee et al., 2002; Calvillo et al., 2004; Patel and McGhee, 2009; Chao et al., 2015) and the sub-basal nerves left in the flap may undergo a degenerative process in early postoperative period (Linna et al., 2000; Nettune and Pflugfelder, 2010). Initially, the regenerating fibers appear as short sub-basal branches, but by three months, they continue to grow and become more elongated. The regenerated nerves that are coming from the stroma below the LASIK interface are unable to cross and interconnect with the remaining nerves inside the flap (Linna et al., 2000). The sub-basal nerve density takes more time to recover in LASIK than in PRK and it may never reach baseline levels, reports show sub-basal nerve density remained significantly lower up to 5 years from surgery (Erie et al., 2005).
Unlike with PRK, IVCM and corneal hypoesthesia do not show a consistent correlation. Maximum reinnervation is seen by IVCM after more than 1 year (Lee et al., 2002; Calvillo et al., 2004; Erie et al., 2005; Chao et al., 2015), while corneal sensitivity is restored far more sooner (Perez-Santonja et al., 1999; Bragheeth and Dua, 2005; Lee et al., 2005; Nejima et al., 2005; Darwish et al., 2007; Li et al., 2013; Wei and Wang, 2013; Kung et al., 2014; Chao et al., 2015; Xia et al., 2016). Corneal sensitivity threshold is at its lowest 1–2 weeks after surgery and returns to near normal from 6 to 16 months (Linna et al., 2000; Lee et al., 2002).
SMILE reinnervation
In SMILE sub-basal fibers are resected at the side cut (2–5 mm), some stromal and sub-basal fibers are damaged by the planar dissection of the cap. Conversely, the nerve fibers that do not penetrate Bowman layer and are outside the lenticule/cap and side cut area, remain untouched (Sekundo et al., 2011). Distinct sub-basal fibers are visible at one week postoperatively and at four weeks, there is significant recovery of sub-basal nerve density, length and number (Li et al., 2013; Agca et al., 2015; Ishii et al., 2015; Liu et al., 2015). Long-term studies are limited due to the recent adoption of this technique; however, there is evidence that the nerves keep regenerating even after 2 years (Vestergaard et al., 2013).
Recovery of corneal sensation begins with 4 weeks and peaks at 6 months but it remains lower than the preoperative level afterwards (He et al., 2015b). Li et al. (2014) have shown that loss of sensation does not correlate with the degree of correction or depth of resection with SMILE surgery.
LASIK versus SMILE
In a recent meta-analysis (Kobashi et al., 2017) that was limited to the first 6 post-operative months, SMILE showed relative superiority over LASIK in both recovery of corneal sensation and IVCM corneal nerve density. At 1 month postoperatively, sub-basal nerve density was significantly higher in SMILE-treated eyes than it was in the LASIK ones. However, no significant difference was detected 6 months postoperatively (weighted mean difference (WMD) = 4.72, 95% confidence interval (CI), 1.10–8.34, P = 0.01). Corneal sensitivity was significantly higher in SMILE studies at both 1 and 6 months (WMD = 11.35 and 3.49; 95% CI, 7.29–15.40 and 1.76–5.21; P = 0.00001 and 0.0001, at 1- and 6-month follow-ups, respectively).
LASIK versus PRK
Perez-Santoja et al. (1999) have investigated the differences among PRK and LASIK reinnervation and corneal sensation. They were able to show a faster recovery of both in the PRK group.
Other considerations
Features of corneal nerve regeneration in all refractive surgeries are sprouting, thinning, beading, neuromas and increased tortuosity of the fibers, which are common to aberrant reinnervation (Latvala et al., 1996a, b; Linna et al., 2000; Vestergaard et al., 2013; Hamrah et al., 2017). Hamrah et al. (2017) have shown a series of patients presenting corneal allodynea or keratoneuralgia after laser refractive surgery. They had found a mean sub-basal nerve density of 1322 ± 103 µm per frame, which was much lower than in controls. This finding is in line with the discrepancy between re-establishment of baseline corneal nerve density and sensation recovery after surgery, as discussed previously. The ocular pain in these patients is associated with a hyper-excitable state of corneal somatosensory pathways (Spierer et al., 2016), implicating the role of neural plasticity during the reinnervation after afferent corneal nerve impairment (Belmonte, 2007; Rosenthal and Borsook, 2012). Direct damage to corneal tissue decreases the firing threshold of nerve sodium channels, resulting in high axonal activity (Ehlers et al., 1995), this, in turn, leads to the involvement of low-threshold fibers that are typically responsible for conducting innocuous stimuli such as touch (MacIver and Tanelian, 1993). The recruitment of low-threshold fibers is thought to be one of the mechanisms of keratoneuralgia (MacIver and Tanelian, 1993).
Keratocyte repopulation and neuron growth factor (NGF) have also been implicated in the reinnervation of the cornea after refractive laser surgery. There is strong in vitro evidence supporting their influence in corneal nerve regeneration (Tan et al., 2006; Ma et al., 2014; Yam et al., 2017; Pan et al., 2018) and further investigations with experimental models are needed in order to clarify their role and potential therapeutic effect on nerve regeneration after refractive surgery.
Keratocyte re-population is different amongst the refractive surgeries: most of the PRK ablation is restricted to the anterior aspect of the stroma, which becomes devoid of stromal cells and leaves mid and posterior stroma keratocytes unharmed (Helena et al., 1998); LASIK ablation is focused on a deeper plane, which results in almost complete keratocyte death and apoptosis (Helena et al., 1998; Mohan et al., 2003). Human corneal nerve fibers have been found to invaginate the cytoplasm of some keratocytes, raising the possibility of a cross talk with neuronal cells. This finding implies that keratocytes may be able to provide nutrients and biological cues for proliferation, leading to renewal of the disrupted nerves at a faster regeneration rate (Yam et al., 2017). Hence, the fact that PRK spares most of the corneal stroma keratocytes may explain the superiority in corneal reinnervation with this technique when compared with LASIK (Helena et al., 1998).
NGF is essential for neuron differentiation, function and survival, but it has also been implicated on the modulation of immune reaction, trophic support, healing of ocular surface, corneal sensitivity and tear film function (Lambiase et al., 2012). Experimental and clinical studies have shown that NGF promotes reinnervation after keratoplasty (Pan et al., 2018), LASIK (Ma et al., 2014) and as an adjunctive treatment for neurotrophic corneal ulcers (Tan et al., 2006). The corneal epithelial breakdown at the ocular surface is associated with a higher concentration of NGF on the ocular surface (Cellini et al., 2006). PRK laser ablation completely removes both the epithelium and basement membrane, delivering a high concentration of cytokines to the stromal keratocytes, which will, in turn, up regulate NGF (Li and Tseng, 1995; You et al., 2000; Lee et al., 2005). In SMILE and LASIK, the basement membrane and epithelium are still intact after the surgery, and they may act as a barrier, preventing NGF produced at the ocular surface, in reaching the stroma (Lee et al., 2005). Therefore, the epithelium and basement membrane breakdown can be additional factors for better reinnervation in PRK than in LASIK. Currently, there are no studies comparing corneal nerve regeneration between PRK and SMILE. However, SMILE preserves the basement membrane and corneal epithelial layer in a similar way as LASIK.
Management
Routine management for nerve damage caused by laser surgery is usually performed with prescription of artificial tears until the corneal sensation returns to near normal (Sacchetti and Lambiase, 2017), but this treatment is insufficient to promote or improve corneal reinnervation. Several substances have been tested as candidates for the latter: cacicol (Alcalde et al., 2015), insulin growth factor-1 (Wang et al., 2014), topical NGF (Joo et al., 2004), pituitary adenylate cyclase-activating polypeptide (Fukiage et al., 2007), pigment-epithelium derived factor (Cortina et al., 2012), platelet rich plasma and FK962 (Yabuta et al., 2012).
To date, the evidence of the beneficial effect of the majority of these drugs is limited to the results of in vivo experimental models (Lambiase et al., 2000; Joo et al., 2004; Cortina et al., 2013; Hyon et al., 2014; Ma et al., 2014; He et al., 2015a) and ex vivo effect on human corneal nerves (Lambiase et al., 2000).
These new therapeutic alternatives to accelerate and/or improve the post-operative reinnervation of the cornea are currently under investigation and the scientific evidence regarding their results is still inconsistent to support their adoption. A recent clinical trial showed some of the benefits with NGF treatment at the post-operative management of refractive surgery (Zhang et al., 2016), however this study comprised a small sample and did not evaluate corneal nerve reinnervation with IVCM or the recovery of sensation. Research protocols with more robust study designs, such as randomized clinical trials, evaluating the effects on the recovery of sensation, sub-basal and sub-bowman nerve density must be carried out to further establish safety and efficacy of the other drugs before implementation in the clinical practice.
Conclusion
The evolution of corneal laser surgery has brought many improvements in visual outcomes, post-operative visual rehabilitation and safety profile, even for high ametropias. Nevertheless, corneal nerve plexus injury is still a major side effect and a concern in these patients. Future research is required in order to establish pre-operative and per-operative strategies to reduce the impact of the lasers on the corneal nerves.
Additional file: Open peer review report 1 (90.8KB, pdf) .
Footnotes
Conflicts of interest: The authors declare they have no conflict of interest related to the following manuscript.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Michele R. Colonna, Universita degli Studi di Messina, Italy.
P-Reviewer: Colonna MR; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45:2333–2336. doi: 10.1167/iovs.03-1366. [DOI] [PubMed] [Google Scholar]
- Agca A, Cankaya KI, Yilmaz I, Yildirim Y, Yasa D, Olcucu O, Demircan A, Demirok A, Yilmaz OF. Fellow eye comparison of nerve fiber regeneration after smile and femtosecond laser-assisted LASIK: a confocal microscopy study. J Refract Surg. 2015;31:594–598. doi: 10.3928/1081597X-20150820-04. [DOI] [PubMed] [Google Scholar]
- Al-Aqaba MA, Alomar T, Miri A, Fares U, Otri AM, Dua HS. Ex vivo confocal microscopy of human corneal nerves. Br J Ophthalmol. 2010;94:1251–1257. doi: 10.1136/bjo.2009.178244. [DOI] [PubMed] [Google Scholar]
- Alcalde I, Inigo-Portugues A, Carreno N, Riestra AC, Merayo-Lloves JM. Effects of new biomimetic regenerating agents on corneal wound healing in an experimental model of post-surgical corneal ulcers. Arch Soc Esp Oftalmol. 2015;90:467–474. doi: 10.1016/j.oftal.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Azar DT, Primack JD. Theoretical analysis of ablation depths and profiles in laser in situ keratomileusis for compound hyperopic and mixed astigmatism. J Cataract Refract Surg. 2000;26:1123–1136. doi: 10.1016/s0886-3350(00)00524-1. [DOI] [PubMed] [Google Scholar]
- Barraquer JI. Keratomileusis. Int Surg. 1967;48:103–117. [PubMed] [Google Scholar]
- Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea. J Refract Surg. 2007;23:598–602. doi: 10.3928/1081-597X-20070601-11. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Schmelz M, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–519. [PubMed] [Google Scholar]
- Benitez-del-Castillo JM, del Rio T, Iradier T, Hernandez JL, Castillo A, Garcia-Sanchez J. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20:30–32. doi: 10.1097/00003226-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, Garcia-Sanchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- Booth AJ, Murray A, Tyers AG. The direct brow lift: efficacy, complications, and patient satisfaction. Br J Ophthalmol. 2004;88:688–691. doi: 10.1136/bjo.2003.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragheeth MA, Dua HS. Corneal sensation after myopic and hyperopic LASIK: clinical and confocal microscopic study. Br J Ophthalmol. 2005;89:580–585. doi: 10.1136/bjo.2004.046888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratto L, Ferrari M, Rama P. Excimer laser intrastromal keratomileusis. Am J Ophthalmol. 1992;113:291–295. doi: 10.1016/s0002-9394(14)71581-8. [DOI] [PubMed] [Google Scholar]
- Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004;45:3991–3996. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- Campos M, Hertzog L, Garbus JJ, McDonnell PJ. Corneal sensitivity after photorefractive keratectomy. Am J Ophthalmol. 1992;114:51–54. doi: 10.1016/s0002-9394(14)77412-4. [DOI] [PubMed] [Google Scholar]
- Carones F, Fiore T, Brancato R. Mechanical vs. alcohol epithelial removal during photorefractive keratectomy. J Refract Surg. 1999;15:556–562. doi: 10.3928/1081-597X-19990901-08. [DOI] [PubMed] [Google Scholar]
- Cellini M, Bendo E, Bravetti GO, Campos EC. The use of nerve growth factor in surgical wound healing of the cornea. Ophthalmic Res. 2006;38:177–181. doi: 10.1159/000092626. [DOI] [PubMed] [Google Scholar]
- Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014;12:32–45. doi: 10.1016/j.jtos.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Chao C, Stapleton F, Zhou X, Chen S, Zhou S, Golebiowski B. Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye. Graefes Arch Clin Exp Ophthalmol. 2015;253:2029–2039. doi: 10.1007/s00417-015-3120-1. [DOI] [PubMed] [Google Scholar]
- Cortina MS, He J, Li N, Bazan NG, Bazan HE. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012;130:76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013;54:4109–4116. doi: 10.1167/iovs.13-12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: prospective study. J Cataract Refract Surg. 2007;33:1515–1521. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Davis JP, Smith AL, Giranda C, Squicciarini M. Laser-induced plasma formation in Xe, Ar, N(2), and O(2) at the first four Nd:YAG harmonics. Appl Opt. 1991;30:4358–4364. doi: 10.1364/AO.30.004358. [DOI] [PubMed] [Google Scholar]
- Dimasi DP, Burdon KP, Craig JE. The genetics of central corneal thickness. Br J Ophthalmol. 2010;94:971–976. doi: 10.1136/bjo.2009.162735. [DOI] [PubMed] [Google Scholar]
- Dohlman TH, Lai EC, Ciralsky JB. Dry eye disease after refractive surgery. Int Ophthalmol Clin. 2016;56:101–110. doi: 10.1097/IIO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- Eguchi H, Hiura A, Nakagawa H, Kusaka S, Shimomura Y. Corneal nerve fiber structure, its role in corneal function, and its changes in corneal diseases. Biomed Res Int 2017. 2017:3242649. doi: 10.1155/2017/3242649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Feng YF, Yu JG, Wang DD, Li JH, Huang JH, Shi JL, Ye T, Wang QM, Zhao YE. The effect of hinge location on corneal sensation and dry eye after LASIK: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2013;251:357–366. doi: 10.1007/s00417-012-2078-5. [DOI] [PubMed] [Google Scholar]
- Frost MH, Schaid DJ, Sellers TA, Slezak JM, Arnold PG, Woods JE, Petty PM, Johnson JL, Sitta DL, McDonnell SK, Rummans TA, Jenkins RB, Sloan JA, Hartmann LC. Long-term satisfaction and psychological and social function following bilateral prophylactic mastectomy. JAMA. 2000;284:319–324. doi: 10.1001/jama.284.3.319. [DOI] [PubMed] [Google Scholar]
- Fukiage C, Nakajima T, Takayama Y, Minagawa Y, Shearer TR, Azuma M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol. 2007;143:255–262. doi: 10.1016/j.ajo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Gatinel D, Hoang-Xuan T, Azar DT. Three-dimensional representation and qualitative comparisons of the amount of tissue ablation to treat mixed and compound astigmatism. J Cataract Refract Surg. 2002a;28:2026–2034. doi: 10.1016/s0886-3350(02)01379-2. [DOI] [PubMed] [Google Scholar]
- Gatinel D, Hoang-Xuan T, Azar DT. Volume estimation of excimer laser tissue ablation for correction of spherical myopia and hyperopia. Invest Ophthalmol Vis Sci. 2002b;43:1445–1449. [PubMed] [Google Scholar]
- Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, Rosenthal P. Corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15:139–151. doi: 10.1016/j.jtos.2016.10.002. [DOI] [PubMed] [Google Scholar]
- He J, Cortina MS, Kakazu A, Bazan HE. The PEDF neuroprotective domain plus DHA induces corneal nerve regeneration after experimental surgery. Invest Ophthalmol Vis Sci. 2015a;56:3505–3513. doi: 10.1167/iovs.15-16755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Huang W, Zhong X. Central corneal sensitivity after small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: a meta-analysis of comparative studies. BMC Ophthalmol. 2015b;15:141. doi: 10.1186/s12886-015-0129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Honigman RJ, Phillips KA, Castle DJ. A review of psychosocial outcomes for patients seeking cosmetic surgery. Plast Reconstr Surg. 2004;113:1229–1237. doi: 10.1097/01.PRS.0000110214.88868.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyon JY, Hose S, Gongora C, Sinha D, O’Brien T. Effect of macrophage migration inhibitory factor on corneal sensitivity after laser in situ keratomileusis in rabbit. Korean J Ophthalmol. 2014;28:170–176. doi: 10.3341/kjo.2014.28.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R, Shimizu K, Igarashi A, Kobashi H, Kamiya K. Influence of femtosecond lenticule extraction and small incision lenticule extraction on corneal nerve density and ocular surface: a 1-year prospective, confocal, microscopic study. J Refract Surg. 2015;31:10–15. doi: 10.3928/1081597X-20141218-01. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, del Cerro M, Liang FQ, Loya N, Aquavella JV. Corneal sensitivity and nerve regeneration after excimer laser ablation. Cornea. 1994;13:225–231. doi: 10.1097/00003226-199405000-00006. [DOI] [PubMed] [Google Scholar]
- Joo MJ, Yuhan KR, Hyon JY, Lai H, Hose S, Sinha D, O’Brien TP. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122:1338–1341. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser-assisted LASIK: meta-analysis. Cornea. 2017;36:85–91. doi: 10.1097/ICO.0000000000000999. [DOI] [PubMed] [Google Scholar]
- Kung JS, Sales CS, Manche EE. Corneal sensation and dry eye symptoms after conventional versus inverted side-cut femtosecond LASIK: a prospective randomized study. Ophthalmology. 2014;121:2311–2316. doi: 10.1016/j.ophtha.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Curr Opin Ophthalmol. 2012;23:296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069. [PubMed] [Google Scholar]
- Latvala T, Linna T, Tervo T. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int Ophthalmol Clin. 1996a;36:21–27. doi: 10.1097/00004397-199603640-00005. [DOI] [PubMed] [Google Scholar]
- Latvala T, Barraquer-Coll C, Tervo K, Tervo T. Corneal wound healing and nerve morphology after excimer laser in situ keratomileusis in human eyes. J Refract Surg. 1996b;12:677–683. doi: 10.3928/1081-597X-19960901-08. [DOI] [PubMed] [Google Scholar]
- Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–3664. [PubMed] [Google Scholar]
- Lee HK, Lee KS, Kim HC, Lee SH, Kim EK. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139:965–971. doi: 10.1016/j.ajo.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- Li M, Zhou Z, Shen Y, Knorz MC, Gong L, Zhou X. Comparison of corneal sensation between small incision lenticule extraction (SMILE) and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;30:94–100. doi: 10.3928/1081597X-20140120-04. [DOI] [PubMed] [Google Scholar]
- Li M, Niu L, Qin B, Zhou Z, Ni K, Le Q, Xiang J, Wei A, Ma W, Zhou X. Confocal comparison of corneal reinnervation after small incision lenticule extraction (SMILE) and femtosecond laser in situ keratomileusis (FS-LASIK) PLoS One. 2013;8:e81435. doi: 10.1371/journal.pone.0081435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linna TU, Vesaluoma MH, Perez-Santonja JJ, Petroll WM, Alio JL, Tervo TM. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. 2000;41:393–397. [PubMed] [Google Scholar]
- Liu M, Zhang T, Zhou Y, Sun Y, Wang D, Zheng H, Liu Q. Corneal regeneration after femtosecond laser small-incision lenticule extraction: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2015;253:1035–1042. doi: 10.1007/s00417-015-2971-9. [DOI] [PubMed] [Google Scholar]
- Lopez de la Fuente C, Sanchez-Cano A, Segura F, Hospital EO, Pinilla I. Evaluation of total corneal thickness and corneal layers with spectral-domain optical coherence tomography. J Refract Surg. 2016;32:27–32. doi: 10.3928/1081597X-20151207-03. [DOI] [PubMed] [Google Scholar]
- Ma K, Yan N, Huang Y, Cao G, Deng J, Deng Y. Effects of nerve growth factor on nerve regeneration after corneal nerve damage. Int J Clin Exp Med. 2014;7:4584–4589. [PMC free article] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993;13:4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- Mohamed-Noriega K, Riau AK, Lwin NC, Chaurasia SS, Tan DT, Mehta JS. Early corneal nerve damage and recovery following small incision lenticule extraction (SMILE) and laser in situ keratomileusis (LASIK) Invest Ophthalmol Vis Sci. 2014;55:1823–1834. doi: 10.1167/iovs.13-13324. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Nejima R, Miyata K, Tanabe T, Okamoto F, Hiraoka T, Kiuchi T, Oshika T. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2005;139:64–71. doi: 10.1016/j.ajo.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. 2010;8:135–145. doi: 10.1016/s1542-0124(12)70224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Pallikaris IG, Siganos DS. Excimer laser in situ keratomileusis and photorefractive keratectomy for correction of high myopia. J Refract Corneal Surg. 1994;10:498–510. [PubMed] [Google Scholar]
- Pan Y, Liu F, Qi X, Hu Y, Xu F, Jia H. Nerve growth factor changes and corneal nerve repair after keratoplasty. Optom Vis Sci. 2018;95:27–31. doi: 10.1097/OPX.0000000000001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- Perez-Santonja JJ, Sakla HF, Cardona C, Chipont E, Alio JL. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomileusis for low myopia. Am J Ophthalmol. 1999;127:497–504. doi: 10.1016/s0002-9394(98)00444-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10:2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7:28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Lambiase A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res. 2017;12:1220–1224. doi: 10.4103/1673-5374.213534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval HP, Donnenfeld ED, Kohnen T, Lindstrom RL, Potvin R, Tremblay DM, Solomon KD. Modern laser in situ keratomileusis outcomes. J Cataract Refract Surg. 2016;42:1224–1234. doi: 10.1016/j.jcrs.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Schmoll T, Unterhuber A, Kolbitsch C, Le T, Stingl A, Leitgeb R. Precise thickness measurements of Bowman’s layer, epithelium, and tear film. Optom Vis Sci. 2012;89:E795–802. doi: 10.1097/OPX.0b013e3182504346. [DOI] [PubMed] [Google Scholar]
- Schwitzer JA, Sher SR, Fan KL, Scott AM, Gamble L, Baker SB. Assessing patient-reported satisfaction with appearance and quality of life following rhinoplasty using the FACE-Q appraisal scales. Plast Reconstr Surg. 2015;135:830e–837e. doi: 10.1097/PRS.0000000000001159. [DOI] [PubMed] [Google Scholar]
- Seiler T. Laser surgery of the cornea. Fortschr Ophthalmol. 1990;87:111–114. [PubMed] [Google Scholar]
- Seiler T, Wollensak J. In vivo experiments with the excimer laser--technical parameters and healing processes. Ophthalmologica. 1986;192:65–70. doi: 10.1159/000309615. [DOI] [PubMed] [Google Scholar]
- Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. 2011;95:335–339. doi: 10.1136/bjo.2009.174284. [DOI] [PubMed] [Google Scholar]
- Solomon KD, Fernandez de Castro LE, Sandoval HP, Biber JM, Groat B, Neff KD, Ying MS, French JW, Donnenfeld ED, Lindstrom RL, Joint LSTF. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116:691–701. doi: 10.1016/j.ophtha.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Sommer B, Zschocke I, Bergfeld D, Sattler G, Augustin M. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29:456–460. doi: 10.1046/j.1524-4725.2003.29113.x. [DOI] [PubMed] [Google Scholar]
- Sonigo B, Iordanidou V, Chong-Sit D, Auclin F, Ancel JM, Labbe A, Baudouin C. In vivo corneal confocal microscopy comparison of intralase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2006;47:2803–2811. doi: 10.1167/iovs.05-1207. [DOI] [PubMed] [Google Scholar]
- Spierer O, Felix ER, McClellan AL, Parel JM, Gonzalez A, Feuer WJ, Sarantopoulos CD, Levitt RC, Ehrmann K, Galor A. Corneal mechanical thresholds negatively associate with dry eye and ocular pain symptoms. Invest Ophthalmol Vis Sci. 2016;57:617–625. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley PF, Tanzer DJ, Schallhorn SC. Laser refractive surgery in the United States Navy. Curr Opin Ophthalmol. 2008;19:321–324. doi: 10.1097/ICU.0b013e3283009ee3. [DOI] [PubMed] [Google Scholar]
- Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25:352–355. doi: 10.1097/01.ico.0000176609.42838.df. [DOI] [PubMed] [Google Scholar]
- Tervo K, Latvala TM, Tervo TM. Recovery of corneal innervation following photorefractive keratoablation. Arch Ophthalmol. 1994;112:1466–1470. doi: 10.1001/archopht.1994.01090230080025. [DOI] [PubMed] [Google Scholar]
- Tervo T, Moilanen J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog Retin Eye Res. 2003;22:339–358. doi: 10.1016/s1350-9462(02)00064-2. [DOI] [PubMed] [Google Scholar]
- Tervo T, Tervo K, Eranko L. Ocular neuropeptides. Med Biol. 1982;60:53–60. [PubMed] [Google Scholar]
- Trokel SL, Srinivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96:710–715. doi: 10.1016/s0002-9394(14)71911-7. [DOI] [PubMed] [Google Scholar]
- Vestergaard AH, Gronbech KT, Grauslund J, Ivarsen AR, Hjortdal JO. Subbasal nerve morphology, corneal sensation, and tear film evaluation after refractive femtosecond laser lenticule extraction. Graefes Arch Clin Exp Ophthalmol. 2013;251:2591–2600. doi: 10.1007/s00417-013-2400-x. [DOI] [PubMed] [Google Scholar]
- Wang C, Peng Y, Pan S, Li L. Effect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett. 2014;558:169–174. doi: 10.1016/j.neulet.2013.10.063. [DOI] [PubMed] [Google Scholar]
- Wei S, Wang Y. Comparison of corneal sensitivity between FS-LASIK and femtosecond lenticule extraction (ReLEx flex) or small-incision lenticule extraction (ReLEx smile) for myopic eyes. Graefes Arch Clin Exp Ophthalmol. 2013;251:1645–1654. doi: 10.1007/s00417-013-2272-0. [DOI] [PubMed] [Google Scholar]
- Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alio J, Marshall J, Huang Y, Song B, Hu L, Zhao Y, Zhu S, Gao R, Bao F, Yu A, Yu Y, Lian H, Huang J. Postoperative efficacy, predictability, safety and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. 2017;178:65–78. [Google Scholar]
- Williams KM, Verhoeven VJ, Cumberland P, Bertelsen G, Wolfram C, Buitendijk GH, Hofman A, van Duijn CM, Vingerling JR, Kuijpers RW, Hohn R, Mirshahi A, Khawaja AP, Luben RN, Erke MG, von Hanno T, Mahroo O, Hogg R, Gieger C, Cougnard-Gregoire A, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. Eur J Epidemiol. 2015;30:305–315. doi: 10.1007/s10654-015-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Zhang J, Wu J, Yu K. Comparison of corneal biological healing after femtosecond LASIK and small incision lenticule extraction procedure. Curr Eye Res. 2016;41:1202–1208. doi: 10.3109/02713683.2015.1107590. [DOI] [PubMed] [Google Scholar]
- Yabuta C, Oka T, Kishimoto Y, Ohtori A, Yoshimatsu A, Azuma M. Topical FK962 facilitates axonal regeneration and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol. 2012;153:651–660. doi: 10.1016/j.ajo.2011.09.029. 660 e651. [DOI] [PubMed] [Google Scholar]
- Yam GH, Williams GP, Setiawan M, Yusoff NZ, Lee XW, Htoon HM, Zhou L, Fuest M, Mehta JS. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci Rep. 2017;7:45396. doi: 10.1038/srep45396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilirmak N, Davis Z, Yoo SH. Refractive surgery (SMILE vs. LASIK vs. Phakic IOL) Int Ophthalmol Clin. 2016;56:137–147. doi: 10.1097/IIO.0000000000000120. [DOI] [PubMed] [Google Scholar]
- You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- Zhang C, Ding H, He M, Liu L, Liu L, Li G, Niu B, Zhong X. Comparison of early changes in ocular surface and inflammatory mediators between femtosecond lenticule extraction and small-incision lenticule extraction. PLoS One. 2016;11:e0149503. doi: 10.1371/journal.pone.0149503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


