Abstract
Background
Esthesioneuroblastoma (ENB) is a rare neuroendocrine tumor. The purpose of this study was to compare Kadish to TNM and Dulgeurov’s modified TNM staging systems and determine the impact of stage on primary surgical treatment selection, margin status, and survival.
Methods
The National Cancer Database (NCDB) was used to identify patients diagnosed with ENB between 2004 to 2015. Patients were excluded based on the ability to properly stage their disease as well as the availability of treatment data.
Results
Eight-hundred eighty-three patients had sufficient data for analysis. On multivariate analysis, age and government insurance were associated with primary surgical treatment, whereas tumor stage, gender, race, hospital type and volume, and comorbidity score were not. Age, CDCC score, hospital volume, and nodal status were found to be predictors of survival. Multivariate analysis controlling for stage failed to demonstrate clear survival differences between staging in both TNM and Kadish systems. T-stage and the presence of regional nodal metastasis were associated with increased risk of positive margins on multivariate analysis.
Conclusion:
Although primary surgical management and positive margins can be predicted by certain patient and tumor factors, clinical staging systems for ENB poorly predict prognosis over a 10-year horizon.
Keywords: Esthesioneuroblastoma, Sinonasal neoplasm, Sinonasal malignancies, NCDB
INTRODUCTION
Esthesioneuroblastoma (ENB) is a rare neuroendocrine tumor thought to originate from the olfactory epithelium within the superior nasal vault.1–3 Studies estimate that ENB constitutes 3% to 6% of all sinonasal malignancies.2 Given its rarity, epidemiologic and staging data has been derived largely from single institution studies and meta-analyses. The most widely accepted, yet unofficial, staging system for ENB is the Kadish system.4 However, other proposed staging systems include the conventional Tumor Node Metastasis (TNM) staging by the American Joint Committee on Cancer (AJCC) as well as a modified version of the TNM classification proposed by Dulguerov.5
Treatment for this tumor typically consists of surgery followed by multimodality adjuvant therapy, either radiation or chemoradiation. Although this treatment algorithm is frequently promoted as the standard of care in the literature, physicians often deviate from it. With no randomized controlled trials and little data on the influence of patient-related factors on outcomes, there continues to be variation in the sequence and utilization of these modalities—surgery, radiation, and chemotherapy – for ENB.
Recent studies have attempted to clarify these issues using national cancer registries. Jethanamest et al examined staging and treatment data for ENB using the Surveillance, Epidemiology, and End Results Program.2 Konuthula et al, on the other hand, examined the prognostic utility of the Kadish staging system using the National Cancer Database (NCDB).6 To date, no study has performed comparisons between staging systems.
The current paper utilizes the National Cancer Database (NCDB) to assess factors predictive of primary surgical approach as well as survival. Additionally, it evaluates the prognostic utility of the Kadish staging system, the traditional TNM classification, and the modified TNM-Dulguerov classification. Lastly, we assessed factors associated with positive margin status.
MATERIALS AND METHODS
Data source
The data source for this paper was the NCDB, a joint program of the Commission on Cancer (CoC) and the American College of Surgeons, which collects hospital-based registry data for patients with invasive cancer diagnoses from over 1,400 CoC-accredited facilities.7,8 The data was used in accordance with the NCDB Participant User Files. This study received an Institutional Review Board Waiver by Memorial Sloan Kettering Cancer Center.
Study cohort
We identified patients with a diagnosis of ENB from 2004 to 2015 who were 18 years of age or older. We filtered the database for the following histologic diagnoses based on the International Classification of Diseases for Oncology, third edition (ICDO-3): olfactory neurogenic tumor (9520), olfactory neurocytoma (9521), olfactory neuroblastoma (9522), and olfactory neuroepithelialoma (9523).9 We included only patients with histologically confirmed diagnoses and those with malignant tumors (i.e. invasion past basement membrane). We excluded patients with anomalous primary sites (i.e. middle ear), with unknown staging, or whose location/extension made it impossible to accurately stage in either Kadish, TNM, or modified TNM systems.10,11 We also excluded patients for whom surgical, radiotherapy, or chemotherapy intervention status was unknown or incorrectly listed (i.e. total laryngectomy), and for whom the sequence of surgery and radiotherapy was unknown.
Potential treatment arms included chemotherapy, radiation, chemo-radiation or surgery followed by chemotherapy, radiation, or chemo-radiotherapy. Definitive surgery was defined as any surgical intervention with intent to cure via craniofacial, endoscopic, or cranio-endoscopic resection. This excluded all excisional and incisional biopsies. We therefore isolated only the patients who underwent surgical intervention with an attempt for negative margins.
Outcomes
The main outcomes were 1) to identify variables that were predictive of a primary surgical treatment and 2) to compare staging systems between Kadish, traditional TNM, and Dulgeurov’s modified-TNM with regard to their prognostic utility. Secondary outcomes included overall survival, margin status, as well as regional and distant metastases.
Tumor characteristics
Tumors were staged according to the Kadish staging system, the “Tumor” component of TNM staging as specified in the TNM Staging of Head and Neck Cancer and Neck Dissection Classification, fourth ed4,10, and the modified TNM classification as proposed by Duelguerov et al. A summary of these staging system can be seen in Table 1. Other tumor-related characteristics included tumor grade and margin status. Grade was assessed according to the Hyams scale (I-IV). Margin status was noted to be ‘positive’ for the presence of macroscopically or microscopically positive margins.
Table 1.
Esthesioneuroblastoma Staging Systems for Tumor
| Kadish Staging | |
|---|---|
| Stage A | Tumor confined to the nasal cavity |
| Stage B | Tumor involves the nasal cavity + one or more paranasal sinuses |
| Stage C | Extension of the tumor beyond the sinonasal cavities and into the paranasl sinuses. Involvement of the cribriform lamina, orbit, skull-base, and intracranial |
| Stage D | Cervical lymph node involvement or distant metastasis |
| AJCC TNM Staging (T only) | |
| Maxillary | |
| T1 | Limited to maxillary sinus mucosa with no erosion or destruction of bone |
| T2 | Causing bone erosion or destruction, including extension into the hard palate and/or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates |
| T3 | Invades any of the following: bone of the posterior wall of the maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses |
| T4a | Invades anterior orbital contents, skin of cheek, pterygoid plates, infratemporal fossa, cribriform plate, sphenoid, or frontal sinuses |
| T4b | Invades any of the following: orbital apex, dura mater, bran, middle cranial fossa, cranial nerves other than V2, nasopharynx, or clivus |
| Nasal cavity/ Ethmoid | |
| T1 | Restricted to any 1 subsite, with or without bony invasion |
| T2 | Invades 2 subsites in a single region or extends to involve an adjacent region within the nasoethmoid complex, with or without bony invasion |
| T3 | Extends to invade the medial wall or floor of the orbit, maxillary sinus, palate, or cribriform plate |
| T4a | Invades any of the following: anterior orbital contents, skin of the nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid, or frontal sinuses |
| T4b | Invades any of the following: orbital apex, dura mater, bran, middle cranial fossa, cranial nerves other than V2, nasopharynx, or clivus |
| Dulguerov Modified TNM Staging (T only) | |
| T1 | Nasal cavity/ paranasal sinuses (not sphenoid or superior most ethmoid) |
| T2 | Includes sphenoid with extension to/erosion of cribriform plate |
| T3 | Extends into orbit or anterior cranial fossa without dural invasion |
| T4 | Tumor involving brain |
Covariates
Patient demographic factors included age, sex, race, insurance status, and facility type. Hospital volume was divided into terciles based on the total number of cases seen from 2004 to 2015; low volume constituted one to two cases, medium volume three to 10 cases, and high volume more than 10 cases over the entire period under study. Facility type was categorized as community cancer centers, comprehensive community cancer centers, academic centers (including National Cancer Institute (NCI) designated comprehensive cancer center), and other.
Analysis
We used Pearson’s chi-square test to compare the association between all available patient, tumor, and hospital characteristics for the binary outcome of definitive surgical resection versus non-surgical treatment. Multivariate analysis of this outcome was performed using a generalized hierarchical linear model with a logit link to evaluate predictors of the binary treatment choice while accounting for clustering of patients within hospitals. All statistically significant variables per the univariate analysis were included in the multivariate analysis, with the addition of descriptors of hospital, tumor, and treatment characteristics. Separate models were generated for both Kadish and T-stage (AJCC nasal cavity); the modified-Dulguerov stage was utilized in the univariate analysis but excluded from all multivariate models in order to avoid redundancy.
Five and 10-year survival for covariates were calculated using Kaplan Meier analysis and statistical significance determined using the log-rank test. Multivariate survival analysis was performed using a cox proportional hazard model with separate models generated for Kadish and T-stage. The multivariate model was generated using all statistically significant variables per Kaplan Meier analysis with the addition of descriptors of hospital, tumor, and treatment characteristics. Distant metastasis was excluded from the multivariate analysis given its low incidence, as was the Dulguerov T-stage again to avoid redundancy.
For margin status, we used Pearson’s chi-square test to compare the association between all available patient, tumor, and hospital characteristics versus the binary outcome of positive margins. Multivariable analysis of this outcome was performed using a generalized hierarchical linear model with a logit link to evaluate predictors of the binary treatment outcome of margin status. Covariates in this analysis included those which might conceptually affect margin status.
For all comparisons, P < 0.05 was considered to be statistically significant. Analyses were conducted using Stata Statistical software (Release 12.1; Stata Inc., College Station, TX).
RESULTS
Demographics, Staging, and Primary Treatment Modality
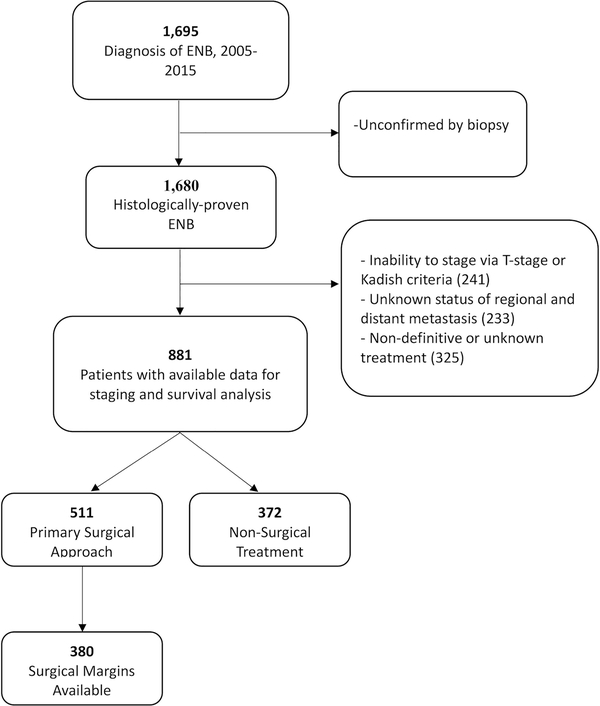
In the NCDB, we identified 1,695 patients with the diagnosis of olfactory neuroblastoma, neurocytoma, or neuroepithelialoma. Of these, 1,680 cases were histologically proven with biopsy and contained information allowing for proper staging. Additional patients were excluded who did not have complete treatment data, leaving 883 patients for analysis [Figure 1]. Approximately 59.9% of the patients were male, and 87.4% were white. The median age was 54 years and demonstrated a unimodal distribution with a peak frequency in the sixth decade of life.
Figure 1.
Flowchart demonstrating inclusion criteria.
Grouping patients by the AJCC criteria for T-stage, a total of 21.9% of the 883 patients were found to be T1, 10.7% were T2, 22.9% were T3, 14.4% were T4a, and 30.1% were T4b. According to the Kadish staging system, 21.9% were Kadish A, 13.4% Kadish B, 55.4% Kadish C, and 9.3% Kadish D. Lastly, according to the Dulguerov system, 36.0% of patients were T1, 21.1% of patients were T2, 15.9% were T3, and 27.1% of patients were T4. Sixty-four (7.3%) of the 883 patients demonstrated regional metastasis, and 23 patients (2.6%) demonstrated distant metastasis [Table 2].
Table 2.
Factors associated with treatment approach.
| Characteristic | Overall |
|---|---|
| No. (Column%) | |
| Sex | |
| Male | 528 (59.9%) |
| Female | 353 (40.1%) |
| Age at Diagnosis | |
| <50 yrs | 323 (36.7%) |
| 50–64 yrs | 355 (40.3%) |
| 65–79 yrs | 176 (19.9%) |
| 80 yrs or more | 27 (3.1%) |
| Race | |
| White | 770 (87.4%) |
| Black | 37 (4.2%) |
| Other | 74 (9.4%) |
| Insurance | |
| Private | 628 (71.4%) |
| Uninsured | 25 (2.8%) |
| Government | 215 (24.4%) |
| Unknown | 13 (1.5%) |
| Hospital Type | |
| Academic/NCI CC | 565 (64.0%) |
| Community | 24 (2.8%) |
| Comp Community | 159 (18.0%) |
| Other/Unknown | 134 (15.2%) |
| Hospital Volume | |
| Low: 1–2 | 285 (32.4%) |
| Medium: 2–10 | 345 (39.2 %) |
| High: >10 | 251 (28.4%) |
| Charles-Deyo Cormibidity Score | |
| 0 | 765 (86.9%) |
| 1 | 96 (10.9%) |
| 2 | 16 (1.8%) |
| 3 | 4 (0.4%) |
| Kadish | |
| A | 193 (21.9%) |
| B | 118 (13.4%) |
| C | 488 (55.4%) |
| D | 82 (9.3%) |
| T-Stage | |
| 1 | 193 (21.9%) |
| 2 | 94 (10.7%) |
| 3 | 202 (22.9%) |
| 4a | 127 (14.4%) |
| 4b | 265 (30.1%) |
| Dulguerov T- Stage | |
| 1 | 317 (36.0%) |
| 2 | 186 (21.1%) |
| 3 | 140 (15.9%) |
| 4 | 238 (27.1%) |
| Lymph Node Status | |
| (+) Lymph Nodes | 64 (7.3%) |
| (−) Lymph Nodes | 817 (92.7%) |
| Distant Metastasis | |
| (+) Metastases | 23 (2.6%) |
| (−) Metastases | 858 (97.4%) |
| Surgical Margin | |
| Negative | 273 (30.9%) |
| Positive | 107 (12.0%) |
| Cannot Assess/ | 130 (14.8%) |
| No surgery | 371 (42.1%) |
| Hyams Grade | |
| 1 | 55 (6.3%) |
| 2 | 180 (20.4%) |
| 3 | 148 (16.9%) |
| 4 | 48 (5.4%) |
| Not stated | 450 (51.0%) |
| Treatment Type | |
| Extirp only | 154 (17.4%) |
| Extirp/RT | 229 (26.1%) |
| Extirp/C | 8 (0.9%) |
| Extirp/CRT | 119 (13.5%) |
| XRT | 240 (27.3%) |
| CRT | 113 (12.8%) |
| C | 18 (2.0%) |
Abbreviations: C, Chemotherapy; CI, confidence interval; CRT, Chemoradiotherapy; ENB, Esthesioneuroblastoma; NCI, National Cancer Institute; NI, not included; NR, not reportable; R, Radiotherapy; Ref, reference.
Definitive surgical treatment was utilized in 57.8%, while 42.2% underwent only non-surgical treatment (other than biopsy). Among all patients, 17.4% underwent surgery alone, 26.1% underwent adjuvant radiation, 13.5% underwent postoperative chemo-radiotherapy, and 0.9% underwent post-operative chemotherapy only. The 42.2% that did not have any surgery included 27.3% who underwent definitive radiation therapy only, 12.8% who underwent definitive chemoradiation, and 2.0% who underwent chemotherapy only [Table 2].
Factors associated with primary surgery
We analyzed factors associated with the use of primary surgical therapy using two different multivariable models, one that accounted for TNM staging, and the other that accounted for Kadish staging. Several factors were associated with the use of a primary surgical approach. Patients 65–79 years of age were less likely to undergo primary surgery compared to those <50 years for the TNM model (OR 0.49; CI 0.28–0.87; P = 0.015) and Kadish model (OR 0.49; CI 0.28–0.86; P = 0.015). Additionally, patients with government insurance were more likely to undergo primary surgery compared to private insurance in both the Kadish (OR 1.87; CI 1.15–3.05, P = 0.012) and TNM (OR 1.85; CI 1.14–3.02, P = 0.013) model. Hospital type and volume, clinical tumor stage, the presence of regional or distant metastasis, tumor grade, race, and comorbidity score did not significantly influence the use of primary surgical intervention [Table 3].
Table 3.
Predictors of primary surgical treatment.
| Characteristic | Primary Surgical Treatment | Multivariate Analysis of Kadish | Multivariate Analysis of AJCC | ||
|---|---|---|---|---|---|
| No. (%) Undergoing surgery | P-Value | P-Value | |||
| N= 511 | |||||
| Sex | P = 0.69 | ||||
| Male | 309 (58.4%) | Ref | Ref | Ref | Ref |
| Female | 202 (57.1%) | 0.93 (0.70–1.23) | P = 0.62 | 0.94 (0.71–1.25) | P = 0.69 |
| Age at Diagnosis | P = 0.75 | ||||
| <50 yrs | 191 (59.1%) | Ref | Ref | Ref | Ref |
| 50–64 yrs | 209 (58.6%) | 0.92 (0.63–1.33) | P = 0.66 | 0.93 (0.64–1.35) | P = 0.71 |
| 65–79 yrs | 97 (55.1%) | 0.49 (0.28–0.86) | P = 0.013 | 0.49 (0.28–0.87) | P = 0.015 |
| 80 yrs or more | 14 (51.9%) | 0.44 (0.18–1.10) | P = 0.080 | 0.46 (0.18–1.13) | P = 0.90 |
| Race | P = 0.16 | ||||
| White | 442 (57.3%) | Ref | Ref | Ref | Ref |
| Black | 19 (51.4%) | 0.84 (0.43–1.66) | P = 0.62 | 0.87 (0.44–1.72) | P = 0.69 |
| Other | 50 (67.6%) | 1.55 (0.92–2.60) | P = 0.10 | 1.56 (0.93–2.63) | P = 0.092 |
| Insurance | P = 0.77 | ||||
| Private | 358 (56.8%) | Ref | Ref | Ref | Ref |
| Uninsured | 16 (64.0%) | 1.38 (0.59–3.23) | P = 0.46 | 1.36 (0.58–3.20) | P = 0.48 |
| Government | 129 (60.0%) | 1.87 (1.15–3.05) | P = 0.012 | 1.85 (1.14–3.02) | P = 0.013 |
| Unknown | 8 (61.5%) | 1.29 (0.40–4.15) | P = 0.67 | 1.27 (0.39–4.07) | P = 0.69 |
| Hospital Type | P = 0. 93 | ||||
| Academic/NCI CC | 327 (57.9%) | Ref | Ref | Ref | Ref |
| Community | 16 (64.0%) | 1.28 (0.53–3.16) | P = 0.58 | 1.30 (0.53–3.19) | P = 0.57 |
| Comp Community | 92 (57.9%) | 0.96 (0.63–1.45) | P = 0.84 | 0.98 (0.64–1.49) | P = 0.92 |
| Other/Unknown | 76 (56.7%) | 0.86 (0.54–1.37) | P = 0.52 | 0.85 (0.53–1.36) | P = 0.51 |
| Hospital Volume | P = 0.55 | ||||
| Low: 1–2 | 165 (57.7%) | Ref | Ref | Ref | Ref |
| Medium: 3–10 | 194 (56.1%) | 1.01 (0.71–1.44) | P = 0.96 | 0.99 (0.70–1.42) | P = 0.97 |
| High: >10 | 152 (60.6%) | 1.20 (0.80–1.80) | P = 0.38 | 1.17 (0.78–1.76) | P = 0.44 |
| Charles-Deyo Cormibidity Score | P = 0.26 | ||||
| 0 | 443 (57.9%) | Ref | Ref | Ref | Ref |
| 1 | 56 (58.3%) | 1.07 (0.68–1.67) | P = 0.77 | 1.95 (0.67–1.64) | P = 0.83 |
| 2 | 7 (43.8%) | 0.56 (0.20–1.55) | P = 0.27 | 0.55 (0.20–1.53) | P = 0.25 |
| 3 | 4 (100%) | NR | NR | NR | NR |
| Kadish | P = 0.34 | ||||
| A | 122 (63.2%) | Ref | Ref | NI | NI |
| B | 70 (59.3%) | 0.85 (0.553–1.38) | P = 0.52 | NI | NI |
| C | 273 (55.8%) | 0.68 (0.48–0.97) | P = 0.34 | NI | NI |
| D | 46 (56.1%) | 0.76 (0.44–1.31) | P = 0.32 | NI | NI |
| T-Stage | P = 0.47 | ||||
| 1 | 122 (63.2%) | NI | NI | Ref | Ref |
| 2 | 52 (55.3%) | NI | NI | 0.71 (0.43–1.18) | P = 0.19 |
| 3 | 117 (57.9%) | NI | NI | 0.78 (0.51–1.18) | P = 0.24 |
| 4a | 68 (53.5%) | NI | NI | 0.64 (0.40–1.02) | P = 0.058 |
| 4b | 152 (57.1%) | NI | NI | 0.73 (0.49–1.08) | P = 0.11 |
| Dulguerov T- Stage | P = 0.70 | ||||
| 1 | 192 (60.6%) | NI | NI | NI | NI |
| 2 | 104 (55.9%) | NI | NI | NI | NI |
| 3 | 81 (57.9%) | NI | NI | NI | NI |
| 4 | 134 (56.1%) | NI | NI | NI | NI |
| Lymph Node Status | P = 0.79 | ||||
| (+) Lymph Nodes | 36 (56.3%) | NI | NI | Ref | Ref |
| (−) Lymph Nodes | 475 (58.0%) | NI | NI | 0.95 (0.56–1.62) | P = 0.86 |
| Distant Metastasis | P = 0.58 | ||||
| (+) Metastases | 12 (52.2%) | NI | NI | Ref | Ref |
| (−) Metastases | 499 (58.0%) | NI | NI | 1.14 (0.48–2.69) | P = 0.77 |
| Hyams Grade | P = 0.89 | ||||
| 1 | 31 (56.4%) | Ref | Ref | Ref | Ref |
| 2 | 105 (58.3%) | 1.18 (0.63–1.21) | P = 0.60 | 1.13 (0.60–2.11) | P = 0.71 |
| 3 | 91 (61.1%) | 1.22 (0.64–2.33) | P = 0.55 | 1.17 (0.62–2.23) | P = 0.63 |
| 4 | 26 (54.2%) | 1.03 (0.46–2.31) | P = 0.95 | 0.99 (0.44–2.23) | P = 0.99 |
| Not stated | 258 (57.3%) | 1.10 (0.62–1.96) | P = 0.75 | 1.05 (0.59–1.88) | P = 0.86 |
Abbreviations: CI, confidence interval; ENB, Esthesioneuroblastoma; NCI, National Cancer Institute; NI, not included; Ref, reference.
Survival
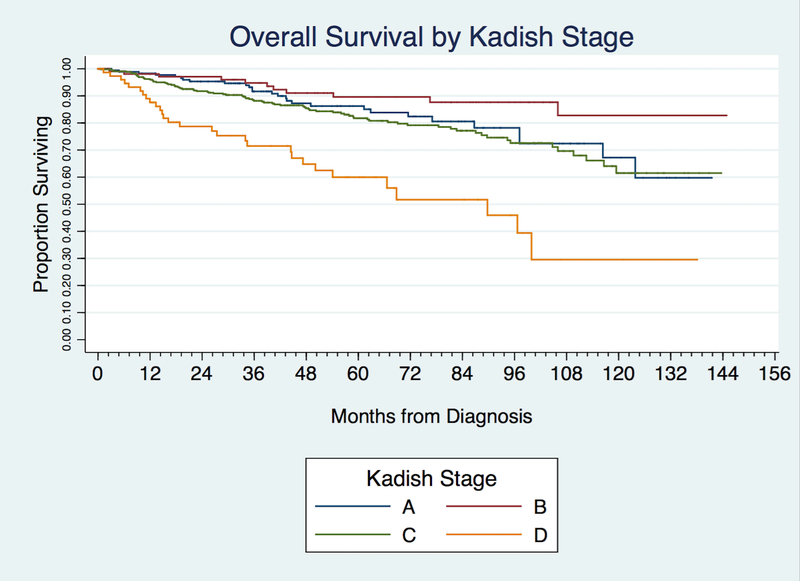
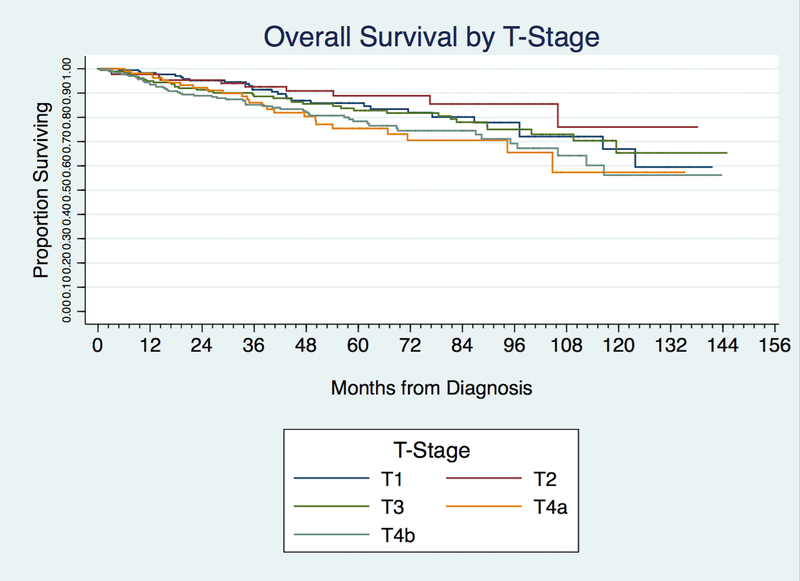
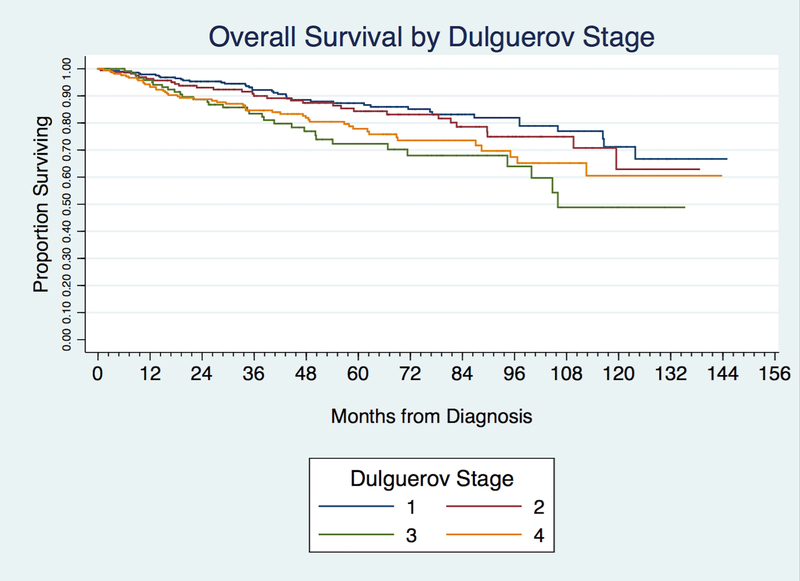
Kaplan-Meier analysis demonstrated an overall 5-year survival of 81.9% and 10-year survival of 63.7%. The 5 and 10-year survival for Kadish staging was 86.3% and 67.2% for Kadish A; 89.6% and 82.7% for Kadish B; 81.8% and 61.5% for Kadish C; and 60.0% and 29.5% for Kadish D. The 5 and 10-year survival for T-stage was 85.9% and 66.9% for T1; 88.9% and 76.0% for T2; 82.8% and 65.4% for T3, 75.4% and 57.3% for T4a, and 78.4% and 56.2% for T4b. The 5 and 10-year survival for the Dulguerov T stage was 87.7% and 71.1% for T1 tumors; 84.3% and 62.9% for T2; 72.3% and 48.9% for T3; and 77.9% and 60.5% for T4 [Table 4].
Table 4.
Predictors of Survival
| Characteristic | 5-Year Survival | 10-Year Survival | Multivariate Analysis of Kadish | Multivariate Analysis of AJCC | ||
|---|---|---|---|---|---|---|
| Total | 449 | 193 | ||||
| Hazard Ratio (C.I.) | P-Value | Hazard Ratio (C.I.) | P-Value | |||
| Overall Survival | 81.9% | 63.7% | ||||
| Sex | P = 0.051 | |||||
| Male | 78.4% | 62.8% | NI | NI | NI | NI |
| Female | 87.3% | 63.4% | NI | NI | NI | NI |
| Age at Diagnosis | P < 0.0001 | |||||
| <50 yrs | 87.1% | 70.0% | Ref | Ref | Ref | Ref |
| 50–64 yrs | 82.3% | 71.3% | 1.83 (1.04–3.20) | P = 0.035 | 1.90 (1.08–3.33) | P = 0.025 |
| 65–79 yrs | 75.5% | 47.0% | 4.72 (2.18–10.22) | P < 0.0001 | 4.53 (2.14–9.64) | P < 0.0001 |
| 80 yrs or more | 50.7% | NR | 18.46 (7.51–45.35) | P <0.0001 | 18.79 (7.64–46.21) | P < 0.0001 |
| Race | P = 0.074 | |||||
| White | 81.4% | 63.3% | NI | NI | NI | NI |
| Black | 78.4% | 56.4% | NI | NI | NI | NI |
| Other | 89.9% | 71.9% | NI | NI | NI | NI |
| Insurance | P = 0.012 | |||||
| Private | 83.8% | 67.1% | Ref | Ref | Ref | Ref |
| Uninsured | 88.9% | 59.2% | 0.72 (0.22–2.35) | P = 0.59 | 0.75 (0.23–2.46) | P = 0.64 |
| Government | 73.7% | 53.5% | 0.79 (0.43–1.44) | P = 0.44 | 0.80 (044–1.44) | P = 0.45 |
| Unknown | NR | NR | 0.17 (0.020–1.41) | P = 0.10 | 0.21 (0.025–1.73) | P = 0.15 |
| Hospital Type | P = 0.97 | |||||
| Academic/NCI CC | 82.2% | 63.7% | Ref | Ref | Ref | Ref |
| Community | 90.7% | NR | 0.52 (0.18–1.51) | P = 0.23 | 0.49 (0.17–1.43) | P = 0.19 |
| Comp Community | 79.0% | 76.0% | 0.99 (0.59–1.66) | P = 0.98 | 0.96 (0.57–1.60) | P = 0.86 |
| Other/Unknown | 82.0% | 53.9% | 2.79 (1.45–5.38) | P = 0.002 | 2.62 (1.36–5.07) | P = 0.004 |
| Hospital Volume | P = 0.029 | |||||
| Low: 1–2 | 77.1% | 51.4% | Ref | Ref | Ref | Ref |
| Medium: 3–10 | 84.8% | 72.2% | 0.57 (0.37–0.87) | P = 0.010 | 0.61 (0.40–0.94) | P = 0.025 |
| High: >10 | 83.5% | 64.7% | 0.69 (0.43–1.10) | P = 0.12 | 0.69 (0.43–1.11) | P = 0.13 |
| Charles-Deyo Cormibidity Score | P = 0.015 | |||||
| 0 | 83.3% | 64.1% | Ref | Ref | Ref | Ref |
| 1 | 80.2% | 64.7% | 1.29 (0.76–2.17) | P = 0.34 | 1.29 (0.77–2.18) | P = 0.33 |
| 2 | 42.9% | NR | 4.57 (2.07–10.11) | P < 0.0001 | 4.32 (1.99–9.42) | P < 0.0001 |
| 3 | NR | NR | 2.66 (0.34–20.76) | P = 0.35 | 2.68 (0.35–20.82) | P = 0.35 |
| Kadish | P < 0.0001 | |||||
| A | 86.3% | 67.2% | Ref | Ref | NI | NI |
| B | 89.6% | 82.7% | 0.64 (0.31–1.30) | P = 0.21 | NI | NI |
| C | 81.8% | 61.5% | 1.50 (0.94–2.41) | P = 0.09 | NI | NI |
| D | 60.0% | 29.5% | 4.20 (2.27–7.77) | P < 0.0001 | NI | NI |
| T-Stage | P = 0.083 | |||||
| 1 | 85.9% | 66.9% | NI | NI | Ref | Ref |
| 2 | 88.9% | 76.0% | NI | NI | 0.87 (0.41–1.84) | P = 0.72 |
| 3 | 82.8% | 65.4% | NI | NI | 1.20 (0.70–2.06) | P = 0.51 |
| 4a | 75.4% | 57.3% | NI | NI | 1.42 (0.80–2.54) | P = 0.24 |
| 4b | 78.4% | 56.2% | NI | NI | 1.66 (0.99–2.79) | P = 0.055 |
| Dulguerov T- Stage | P = 0.0028 | |||||
| 1 | 87.3% | 71.1% | NI | NI | NI | NI |
| 2 | 84.3% | 62.9% | NI | NI | NI | NI |
| 3 | 72.3% | 48.9% | NI | NI | NI | NI |
| 4 | 77.9% | 60.5% | NI | NI | NI | NI |
| Lymph Node Status | P = 0.0002 | |||||
| (+) Lymph Nodes | 68.3% | 31.4% | NI | NI | Ref | Ref |
| (−) Lymph Nodes | 82.9% | 65.9% | NI | NI | 0.39 (0.23–0.66) | P = 0.001 |
| Distant Metastasis | P < 0.0001 | |||||
| (+) Metastases | 36.4% | NR | NI | NI | NI | NI |
| (−) Metastases | 83.3% | 64.8% | NI | NI | NI | NI |
| Hyams Grade | P = 0.064 | |||||
| 1 | 88.4% | 81.0% | Ref | Ref | Ref | Ref |
| 2 | 86.4% | 59.4% | 0.72 (0.26–1.99) | P = 0.53 | 0.85 (0.31–2.31) | P = 0.75 |
| 3 | 71.3% | 69.0% | 1.06 (0.40–2.85) | P = 0.90 | 1.34 (0.50–3.59) | P = 0.56 |
| 4 | 73.5% | 66.8% | 0.97 (0.31–2.99) | P = 0.95 | 1.31 (0.43–4.02) | P = 0.63 |
| Not stated | 83.8% | 61.1% | 0.89 (0.35–2.27) | P = 0.80 | 1.06 (0.41–2.71) | P = 0.90 |
Abbreviations: C, Chemotherapy; CI, confidence interval; CRT, Chemoradiotherapy; ENB, Esthesioneuroblastoma; NCI, National Cancer Institute; NI, not included; NR, not reportable; R, Radiotherapy; Ref, reference.
Cox regression revealed poorer overall survival with age > 50 years for both the TNM model (50–64 years: HR 1.90, CI 1.08–3.33, P = 0.025; 65–79 years: HR 4.53, CI 2.14–9.64, P < 0.0001, ≥80 years: HR 18.79, CI 7.64–46.21, P < 0.001) and the Kadish model (50–64 years: HR 1.83, CI 1.04–3.20, P = 0.035; 65–79 years: HR 4.72, CI 2.18–10.22, P < 0.0001, ≥80 years: HR 18.46, CI 7.51–45.35, P < 0.0001). A Charlson-Deyo score of 2 portended decreased survival in both the TNM (HR 4.57; CI 2.07–10.11; P < 0.0001) and Kadish models (HR 4.32; CI 1.99–9.42; P<0.0001). Medium-volume centers were associated with a decreased rate of mortality compared to low-volume hospitals for both the Kadish model (HR 0.57, CI 0.37–0.87, P = 0.010) and the TNM model (HR 0.61, CI 0.40–0.94, P = 0.25). Negative node-status resulted in a lower likelihood of mortality compared with the presence of loco-regional metastasis (HR 0.39, CI 0.23–0.66, P = 0.001). Race, insurance, hospital type, and Hyams grade did not have statistically significant correlations to survival [Table 4]. Treatment modalities were too heterogenous and with a small n to allow for effective comparisons.
Kaplan Meier analysis and log-rank unadjusted, pair-wise comparison between Kadish stages demonstrated no significant differences in survival between Kadish A and B (P =0.091) and Kadish A and C (P = 0.36) [Figure 2 and Table 5]. Using unadjusted comparisons between AJCC T-stages, there was no difference between T1 and T2, T1 and T3, as well as T3 and T4 tumors. [Figure 3 and Table 5]. Similarly, in Dulguerov T-stage there was no difference between T1 and T2 or T3 and T4 lesions [Figure 4 and Table 5]. Regression analysis demonstrated poorer survival only when comparing Kadish stage A and D (HR 4.20, CI 2.28–7.77–7.83, P < 0.0001). Within the AJCC staging system, there was no association between T-stages and survival [Table 5].
Figure 2.
Kaplan-Meier curve demonstrating overall survival by Kadish stage.
Table 5.
Log-rank comparison between Dulguerov stages.
| Kadish State | A | B | C | D |
|---|---|---|---|---|
| A | P = 0.091 | P = 0.36 | P<0.0001 | |
| B | P = 0.014 | P<0.0001 | ||
| C | P<0.0001 | |||
| D |
| Table 5A. Log-rank comparison between Kadish stages. | |||||
|---|---|---|---|---|---|
| AJCC T-Stage | 1 | 2 | 3 | 4a | 4b |
| 1 | P = 0.31 | P = 0.74 | P = 0.11 | P = 0.08 | |
| 2 | P = 0.19 | P = 0.027 | P = 0.024 | ||
| 3 | P = 0.22 | P = 0.18 | |||
| 4a | P = 0.88 | ||||
| 4b | |||||
| Table 5B. Log-rank comparison between AJCC T-stages. | ||||
|---|---|---|---|---|
| Dulguerov T-Stage | 1 | 2 | 3 | 4 |
| 1 | P = 0.41 | P = 0.0006 | P = 0.0069 | |
| 2 | P = 0.021 | P = 0.12 | ||
| 3 | P = 0.36 | |||
| 4 | ||||
Figure 3.
Kaplan-Meier curve demonstrating overall survival by T-stage.
Figure 4.
Kaplan-Meier curve demonstrating overall survival by Dulguerov stage.
Predictors of positive margins after surgery
In total, only 380 of the 511 patients undergoing surgery had margin status available for analysis. Of these 380 patients, 28.2% had positive margins. Several factors were associated with an increased risk of positive margins after surgery. In the multivariable analyses, patients undergoing surgery at a comprehensive community hospital were more likely to have positive margins compared with an academic or NCI-designated institution. This was true of both the TNM model (OR 2.16; CI 1.05–4.41, P = 0.035) and the Kadish model (OR 2.10, CI 1.04–4.27, P = 0.039) [Table 6]. Increasing stage (TNM stage and Kadish) was also associated with an increased risk of positive margins. In the TNM model, T-stage ≥ 3 (T3: OR 2.22, CI 1.03–4.77, P = 0.041; T4a: OR 3.56, CI: 1.46–8.69, P = 0.005; T4b: OR 4.70, CI 2.25–9.81, P < 0.0001) and positive nodal metastasis (OR 0.32, CI 0.13–0.78, P = 0.012) predicted positive margins [Table 6]. Among those with positive margins, 24.3% had no adjuvant treatment, 41.1% underwent postoperative radiation, 31.8% underwent postoperative chemoradiation, and 2.8% received postoperative chemotherapy (data not shown). Among those with negative margins, 68.5% received adjuvant therapy with 48.7% receiving postoperative radiation (data not shown).
Table 6.
Predictors of surgical margin status.
| Characteristic | Positive Margins | Multivariate Analysis of Kadish | Multivariate Analysis of AJCC | ||
|---|---|---|---|---|---|
| No./Total Patients (%) | P-Value | P-Value | |||
| Overall | 107/380 (28.2%) | ||||
| Sex | P = 0.91 | ||||
| Male | 64/229 (28.0%) | NI | NI | NI | NI |
| Female | 43/151 (28.5%) | NI | NI | NI | NI |
| Age at Diagnosis | P = 0.77 | ||||
| <50 yrs | 43/141 (30.5%) | NI | NI | NI | NI |
| 50–64 yrs | 41/155 (26.5%) | NI | NI | NI | NI |
| 65–79 yrs | 19/73 (26.0%) | NI | NI | NI | NI |
| 80 yrs or more | 4/11 (36.4%) | NI | NI | NI | NI |
| Race | P = 0.081 | ||||
| White | 95/330 (28.8%) | NI | NI | NI | NI |
| Black | 7/16 (43.8%) | NI | NI | NI | NI |
| Other | 5/34 (14.7%) | NI | NI | NI | NI |
| Insurance | P = 0.36 | ||||
| Private | 77/268 (28.7%) | NI | NI | NI | NI |
| Uninsured | 1/12 (8.3%) | NI | NI | NI | NI |
| Government | 28/93 (30.1%) | NI | NI | NI | NI |
| Unknown | 1/7 (14.3%) | NI | NI | NI | NI |
| Hospital Type | P = 0.46 | ||||
| Academic/NCI CC | 64/250 (25.6%) | Ref | Ref | Ref | Ref |
| Community | 3/11 (28.3%) | 1.57 (0.36–6.89) | P = 0.55 | 1.68 (0.37–7.52) | P = 0.50 |
| Comp. Community | 21/63 (33.3%) | 2.10 (1.04–4.27) | P = 0.039 | 2.16 (1.05–4.41) | P = 0.035 |
| Other/Unknown | 19/56 (28.2%) | 1.34 (0.69–2.58) | P = 0.39 | 1.46 (0.75–2.83) | P = 0.27 |
| Hospital Volume | P = 0.56 | ||||
| Low: 1–2 | 20/114 (26.3%) | Ref | Ref | Ref | Ref |
| Medium: 3–10 | 44/140 (31.4%) | 1.21 (0.65–2.25) | P = 0.55 | 1.28 (0.68–2.40) | P = 0.44 |
| High: >10 | 33/126 (26.2%) | 1.08 (0.55–2.15) | P = 0.82 | 1.08 (0.54–2.19) | P = 0.83 |
| Charles-Deyo Cormibidity Score | P = 0.77 | ||||
| 0 | 90/330 (27.3%) | NI | NI | NI | NI |
| 1 | 15/43 (34.9%) | NI | NI | NI | NI |
| 2 | 1/4 (25.0%) | NI | NI | NI | NI |
| 3 | 1/3 (33.3%) | NI | NI | NI | NI |
| Kadish | P < 0.0001 | ||||
| A | 13/92 (14.1%) | Ref | Ref | NI | NI |
| B | 12/56 (21.4%) | 1.52 (0.63–3.67) | P = 0.35 | NI | NI |
| C | 65/200 (32.5%) | 3.09 (1.57–6.08) | P = 0.001 | NI | NI |
| D | 17/32 (53.1%) | 8.39 (3.20–22.0) | P < 0.0001 | NI | NI |
| T-Stage | P < 0.0001 | ||||
| 1 | 13/91 (14.3%) | NI | NI | Ref | Ref |
| 2 | 7/42 (16.7%) | NI | NI | 1.30 (0.47–3.59) | P = 0.062 |
| 3 | 27/98 (27.6%) | NI | NI | 2.22 (1.03–4.77) | P = 0.041 |
| 4a | 16/44 (36.4%) | NI | NI | 3.56 (1.46–8.69) | P = 0.005 |
| 4b | 44/105 (41.9%) | NI | NI | 4.70 (2.25–9.81) | P < 0.0001 |
| Dulguerov T- Stage | P<0.0001 | ||||
| 1 | 25/150 (16.7%) | NI | NI | NI | NI |
| 2 | 23/83 (27.7%) | NI | NI | NI | NI |
| 3 | 16/56 (28.6%) | NI | NI | NI | NI |
| 4 | 43/91 (47.3%) | NI | NI | NI | NI |
| Lymph Node Status | P = 0.003 | ||||
| (+) Lymph Nodes | 13/24 (54.2%) | NI | NI | Ref | Ref |
| (−) Lymph Nodes | 94/356 (26.4%) | NI | NI | 0.32 (0.13–0.78) | P = 0.012 |
| Distant Metastasis | P = 0.17 | ||||
| (+) Metastases | 4/8 (50.0%) | NI | NI | NI | NI |
| (−) Metastases | 103/372 (28.2%) | NI | NI | NI | NI |
| Hyams Grade | P = 0.61 | ||||
| 1 | 9/27 (33.3%) | Ref | Ref | Ref | Ref |
| 2 | 23/74 (31.1%) | 0.83 (0.31–2.22) | P = 0.71 | 0.82 (0.30–2.23) | P = 0.70 |
| 3 | 22/67 (32.8%) | 0.88 (0.32–2.38) | P = 0.80 | 0.81 (0.29–2.22) | P = 0.68 |
| 4 | 6/20 (30.0%) | 0.62 (0.16–2.40) | P = 0.49 | 0.57 (0.15–2.21) | P = 0.42 |
| Not stated | 47/192 (24.5%) | 0.55 (0.22–1.39) | P = 0.21 | 0.53 (0.21–1.33) | P = 0.18 |
Abbreviations: CI, confidence interval; ENB, Esthesioneuroblastoma; NCI, National Cancer Institute; NI, not included; Ref, reference.
DISCUSSION
Esthesioneuroblastoma is an exceedingly rare tumor, making it very difficult to study. Still, multiple institutions have published their own experience with the disease, analyzing data over extended periods of time in order to accumulate a larger sample size.12–17 Other studies have utilized existing literature for meta-analyses or even multi-institutional databases to obtain a larger sample size. 1–4,11,13–16,18–31 Recent studies have examined different aspects of the ENB population in the NCDB.6,32 To our knowledge, this is the first study to evaluate the comparison between clinical staging systems for ENB, as well as identify factors influencing the use of primary surgical treatment and factors affecting margin status.
Previous studies have evaluated the utility of the Kadish staging system.2,5,6,28,33–35 While some have validated the prognostic utility of the Kadish staging system, others have been unable to prove an association with staging. Most recently, Konuthula et al examined the NCDB from 2004–2013 and found a difference in hazard ratios when comparing Kadish A, B, and C tumors to Kadish D tumors.6 Upon further assessment, they noted a paradoxical improvement in survival from patients with Kadish A tumors to patients with Kadish B tumors which they attributed to a multitude of factors including limitations of the NCDB as well as selection bias; they also suggest that the discrepancy may also be due to the lack prognostic ability of the Kadish system itself.
Our study found a similar increase in survival between Kadish A and B. However, when compared in both an unadjusted as well as adjusted manner, this survival difference was not found to be statistically significant. Using Kadish A as a reference, we found a significant difference in survival only when comparing Kadish A and Kadish D on multivariate analysis. An unadjusted comparison demonstrated a significant survival difference between all stages except Kadish A and B and Kadish A and C. This finding may reflect a treatment bias with smaller tumors undergoing a less radical resection and larger tumors, extending beyond the nasal cavity, undergoing a wider, more radical resection. In addition, these differences could be secondary to the higher likelihood of adjuvant therapy for larger tumors. Our data demonstrates that tumors underwent adjuvant radiation or chemoradiation following surgical resection in 28.5 % of Kadish A staged lesions, 38.9% of Kadish B, 42.8% of Kadish C, and 46.3% of Kadish D (data not shown). On the other hand, when examining the SEER database over a 30-year period, Jethanamest et al noted an unadjusted stepwise difference in survival between all Kadish stages except Kadish C.2
Our data did not demonstrate much utility for T-stage in assessing prognosis as evidenced by both unadjusted pairwise comparisons as well as multi-variate analysis. The Dulguerov T-stage did show prognostic utility based upon the 5 and 10-year survival value; however, these values were not found to be significant on unadjusted comparison. Of note, the presence of positive lymph nodes indicated a poorer prognosis based on Kaplan Meier analysis as well as regression analysis. This finding likely accounts for the survival difference found between Kadish A and Kadish D as the latter represents patients with regional or distant metastasis. Previous studies have demonstrated similar findings. Banuchi et al found a poor prognosis for patients both with nodal metastasis at presentation as well as those with nodal recurrence after treatment of the primary site.36 The cause of death in these patients, however, was distant failure, not uncontrollable loco-regional disease, indicating that the presence of nodal metatasis may be a marker of aggressive tumor biology.
It has also been noted that Hyams grade may be a confounder when considering Kadish stage.37 An analysis by Kane et al noted that Hyams grade was independently associated with survival and further, that Kadish stage was no longer predictive of survival when controlling for Hyams grade.30 Our study did not find an association between Hyams grade and survival on the Kadish model or TNM model, even when grouping tumors into a binary classification of low grade and high grade (data not shown).
The covariates associated with treatment selection for surgery included age and insurance status. Patients 65–79 years old were less likely to undergo primary surgical treatment. Although patients ≥80 years were less likely to undergo primary surgery compared with patients <50 years, it did not achieve statistical significance, likely due to the smaller number of patients in this sub-category. Age also demonstrated an association with survival as age ≥ 50 years portended a poorer survival outcome. Previous studies have not commented on the association of these covariates with treatment choice.
Treatment center, stage, and the presence of regional metastasis predicted the presence of positive surgical margins. Surgery performed at community as well as comprehensive community centers was more likely to result in positive margins compared with surgery performed at an academic of NCI center. Of note, the likelihood ratio for community centers was not statistically significant, most likely due to the small number of patient treatments at community facilities. Patients treated at medium and high-volume centers also tended to have a lower rate of mortality. While the hazard ratio for high volume centers did not reach statistical significance, it is possible that there may be an element of selection bias with more advanced tumors presenting to higher volume centers.
Other studies evaluating head and neck malignancy have also observed improved outcomes for patients treated at comprehensive, high volume centers. Specifically, they have demonstrated a difference in treatment outcomes (ie positive margins, poorer survival, etc) between lower volume and non-academic medical centers compared with larger volume and academic centers.38,39 Unsurprisingly, advanced Kadish tumors (C and D) as well as advanced T-stage (T3, T4a, and T4b) and N+ tumors all had a greater likelihood of resultant positive margins, indicating that the invasion of key anatomic sites as well as overall size portended a smaller likelihood of resection of the entirety of the tumor with removal of adjacent normal tissue.
The most important factor to consider when assessing any study of ENB is the duration of the study. Although our treatment timeline examined an additional two years of data compared to that of Konuthula et al, it is not as robust compared to 30-year periods as can be seen in a SEER study.3,6 However, such long term follow-up can demonstrate diminished outcomes as a result of changes in primary treatment paradigms as well as evolution in technology of both surgical and non-surgical treatment. For example, Platek et al. reported a 35% 5 year survival rate for patients treated with radiation therapy alone in the SEER study, compared to this study’s 86.9% (data not shown).3 The focus of this study was assessment of patient, tumor, and non-tumor factors that impact utilization of a primary surgical modality as well as the utility of the three commonly used staging systems in the modern era. Therefore, the 10 year horizon in this study is most appropriate for the questions assessed. In addition, recurrence typically occurs from 5 to 10 years postoperatively.19 Ow et al noted that 46% of their population experienced disease recurrence with a median time to recurrence of 6.9 years.19 Nevertheless, the NCDB provides other notable advantages to the SEER database including a larger sample size, an increased breadth of tumor characteristics, and more comprehensive treatment data.
A major pitfall of the NCDB is that only overall survival is recorded. Disease-free survival, disease-specific survival, and information regarding recurrence is not tabulated. As such, it was not possible to assess the progression of the disease in response to treatment. Furthermore, the NCDB only began tracking the method of surgical approach in 2009. As a result of incomplete data, we could not complete a full assessment of the method of surgical approach (open versus endoscopic) and its effect on survival. Other studies have addressed this topic, with the notable limitation of selection bias.18,40 Future avenues of research should continue to monitor outcomes for ENB over longer periods of time and survival outcomes for endoscopic approaches.
Lastly, as with all large, database-driven studies, we depend upon information that has been entered by registrars who often must interpret operative reports and physician documentation. Errors in this process may affect the data and ultimately, conclusions that may be drawn from the data.
CONCLUSION
This study represents the first analysis of multiple staging systems for ENB using the NCDB. The AJCC TNM, Dulguerov’s modified TNM, and Kadish staging systems all poorly depict patient prognosis over a 10-year horizon. It is clear that longer term studies are needed to assess the durability of these staging systems in the setting of this insidious pathology.
Synopsis: Esthesioneuroblastoma is a rare neuroendocrine tumor with multiple clinical staging systems. This study demonstrates that these staging systems poorly predict prognosis over 10 years.
Acknowledgments
Financial Disclosures: This research was supported by a NIH/NCI Cancer Center Support Grant, Grant number: P30 CA008748. Additionally, Nancy Y. Lee sits on the advisory board of Pfizer, Merck, Merck-Serono, Lily, and Sanofi Aventis.
Footnotes
Conflicts of Interest: None
Other: Presented at 2016 COSM Meeting San Diego, California. All Authors have approved the final manuscript and attest to the integrity of the original data and the analysis reported.
REFERENCES
- 1.Oskouian RJ Jr., Jane JA Sr., Dumont AS, Sheehan JM, Laurent JJ, Levine PA. Esthesioneuroblastoma: clinical presentation, radiological, and pathological features, treatment, review of the literature, and the University of Virginia experience. Neurosurg Focus 2002; 12:e4. [DOI] [PubMed] [Google Scholar]
- 2.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg 2007; 133:276–280. [DOI] [PubMed] [Google Scholar]
- 3.Platek ME, Merzianu M, Mashtare TL et al. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol 2011; 6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery 1993; 32:706–714; discussion 714–705. [DOI] [PubMed] [Google Scholar]
- 5.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. Laryngoscope 1992; 102:843–849. [DOI] [PubMed] [Google Scholar]
- 6.Konuthula N, Khan MN, Parasher A et al. The presentation and outcomes of mucosal melanoma in 695 patients. Int Forum Allergy Rhinol 2017; 7:99–105. [DOI] [PubMed] [Google Scholar]
- 7.Laske RD, Roosli C, Chatzimichalis MV, Sim JH, Huber AM. The influence of prosthesis diameter in stapes surgery: a meta-analysis and systematic review of the literature. Otol Neurotol 2011; 32:520–528. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008; 15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. International classification of diseases for oncology. Geneva: WHO, 2000. [Google Scholar]
- 10.Deschler DG MM, Smith RV, eds. Quick Reference Guide to TNM Staging of Head and Neck Cancer and Neck Dissection Classification. Alexandria, VA: American Academy of Otolaryngology–Head and Neck Surgery Foundation, 2014. [Google Scholar]
- 11.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 1976; 37:1571–1576. [DOI] [PubMed] [Google Scholar]
- 12.McElroy EA Jr., Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery 1998; 42:1023–1027; discussion 1027–1028. [DOI] [PubMed] [Google Scholar]
- 13.Herr MW, Sethi RK, Meier JC et al. Esthesioneuroblastoma: an update on the massachusetts eye and ear infirmary and massachusetts general hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. J Neurol Surg B Skull Base 2014; 75:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loy AH, Reibel JF, Read PW et al. Esthesioneuroblastoma: continued follow-up of a single institution’s experience. Arch Otolaryngol Head Neck Surg 2006; 132:134–138. [DOI] [PubMed] [Google Scholar]
- 15.Ward PD, Heth JA, Thompson BG, Marentette LJ. Esthesioneuroblastoma: Results and Outcomes of a Single Institution’s Experience. Skull Base 2009; 19:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimmer J, Lund VJ, Beale T, Wei WI, Howard D. Olfactory neuroblastoma: a 35-year experience and suggested follow-up protocol. Laryngoscope 2014; 124:1542–1549. [DOI] [PubMed] [Google Scholar]
- 17.Diaz EM Jr., Johnigan RH 3rd, Pero C et al. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck 2005; 27:138–149. [DOI] [PubMed] [Google Scholar]
- 18.Devaiah AK, Andreoli MT. Treatment of esthesioneuroblastoma: a 16-year meta-analysis of 361 patients. Laryngoscope 2009; 119:1412–1416. [DOI] [PubMed] [Google Scholar]
- 19.Ow TJ, Hanna EY , Roberts DBet al. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck 2014; 36:524–530. [DOI] [PubMed] [Google Scholar]
- 20.Berger L, Luc H, Richard R. L’esthesioneuroepitheliome olfactif. Bull Assoc Fr Etude Cancer 1924; 13:410–420. [Google Scholar]
- 21.Skolnik EM, Massari FS, Tenta LT. Olfactory neuroepithelioma. Review of the world literature and presentation of two cases. Arch Otolaryngol 1966; 84:644–653. [DOI] [PubMed] [Google Scholar]
- 22.Elkon D, Hightower SI, Lim ML, Cantrell RW, Constable WC. Esthesioneuroblastoma. Cancer 1979; 44:1087–1094. [DOI] [PubMed] [Google Scholar]
- 23.O’Conor GT Jr. Drake CR, Johns ME, Cail WS, Winn HR, Niskanen E. Treatment of advanced esthesioneuroblastoma with high-dose chemotherapy and autologous bone marrow transplantation. A case report. Cancer 1985; 55:347–349. [DOI] [PubMed] [Google Scholar]
- 24.Foote RL, Morita A, Ebersold MJ et al. Esthesioneuroblastoma: the role of adjuvant radiation therapy. Int J Radiat Oncol Biol Phys 1993; 27:835–842. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharyya N, Thornton AF, Joseph MP, Goodman ML, Amrein PC. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. Results in 9 cases. Arch Otolaryngol Head Neck Surg 1997; 123:34–40. [DOI] [PubMed] [Google Scholar]
- 26.Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res 1997; 17:2683–2706. [PubMed] [Google Scholar]
- 27.Resto VA, Eisele DW, Forastiere A, Zahurak M, Lee DJ, Westra WH. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck 2000; 22:550–558. [DOI] [PubMed] [Google Scholar]
- 28.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol 2001; 2:683–690. [DOI] [PubMed] [Google Scholar]
- 29.Monroe AT, Hinerman RW, Amdur RJ, Morris CG, Mendenhall WM. Radiation therapy for esthesioneuroblastoma: rationale for elective neck irradiation. Head Neck 2003; 25:529–534. [DOI] [PubMed] [Google Scholar]
- 30.Kane AJ, Sughrue ME, Rutkowski MJ et al. Posttreatment prognosis of patients with esthesioneuroblastoma. J Neurosurg 2010; 113:340–351et al. [DOI] [PubMed] [Google Scholar]
- 31.Patel SG, Singh B, Stambuk HE et al. Craniofacial surgery for esthesioneuroblastoma: report of an international collaborative study. J Neurol Surg B Skull Base 2012; 73:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robin TP, Jones BL, Gordon OM et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modesto A, Blanchard P, Tao YG et al. Multimodal treatment and long-term outcome of patients with esthesioneuroblastoma. Oral Oncol 2013; 49:830–834. [DOI] [PubMed] [Google Scholar]
- 34.Ozsahin M, Gruber G, Olszyk O et al. Outcome and prognostic factors in olfactory neuroblastoma: a rare cancer network study. Int J Radiat Oncol Biol Phys 2010; 78:992–997. [DOI] [PubMed] [Google Scholar]
- 35.McLean JN, Nunley SR, Klass C, Moore C, Muller S, Johnstone PA. Combined modality therapy of esthesioneuroblastoma. Otolaryngol Head Neck Surg 2007; 136:998–1002. [DOI] [PubMed] [Google Scholar]
- 36.Banuchi VE, Dooley L, Lee NY et al. Patterns of regional and distant metastasis in esthesioneuroblastoma. Laryngoscope 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malouf GG, Casiraghi O, Deutsch E, Guigay J, Temam S, Bourhis J. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer 2013; 49:1324–1334. [DOI] [PubMed] [Google Scholar]
- 38.Cracchiolo JR, Baxi SS, Morris LG et al. Increase in primary surgical treatment of T1 and T2 oropharyngeal squamous cell carcinoma and rates of adverse pathologic features: National Cancer Data Base. Cancer 2016; 122:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cracchiolo JR, Patel K, Migliacci JC et al. Factors associated with a primary surgical approach for sinonasal squamous cell carcinoma. J Surg Oncol 2018; 117:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song CM, Won TB, Lee CH, Kim DY, Rhee CS. Treatment modalities and outcomes of olfactory neuroblastoma. Laryngoscope 2012; 122:2389–2395. [DOI] [PubMed] [Google Scholar]