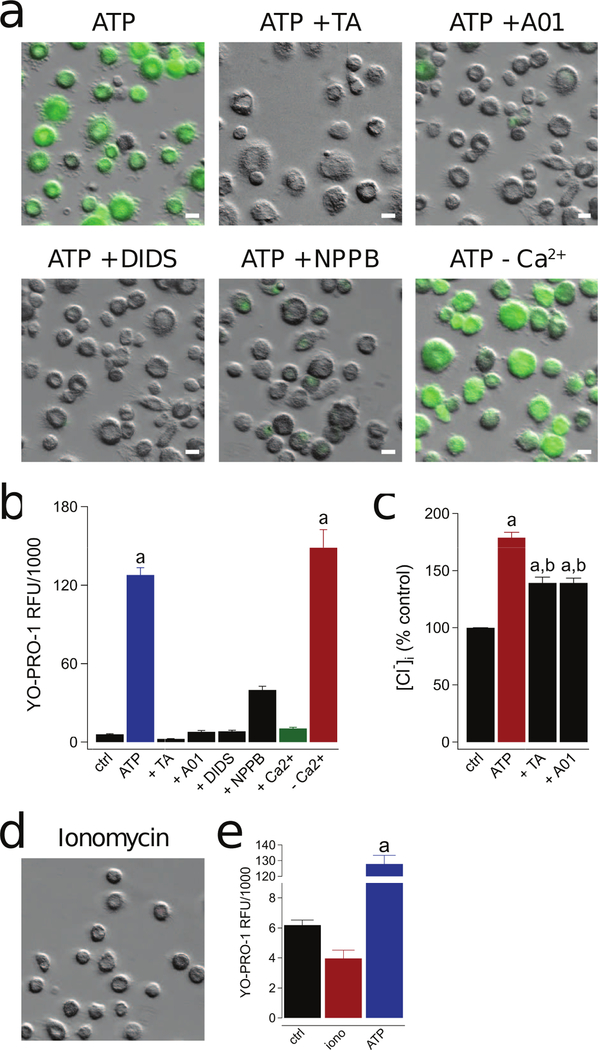
Figure 5. P2X7R-mediated activation of a Cl− channel is responsible for human macrophage permeabilization.
(a) Representative images of macrophages pre-incubated for 30 min with the non-selective Cl− channel inhibitors tannic acid (TA, 20 µM), A01 (40 µM), DIDS (100 µM), and NPPB (0.5 mM). YO-PRO-1 uptake was measured after 15 min in the presence of ATP (2 mM) in normal ECS at 37°C. The independence of YO-PRO-1 uptake on Ca2+ flux was assessed by loading the cells with 10 µM BAPTA-AM and measuring dye uptake in Ca2+ free ECS with 1 mM EDTA (-Ca2+). Scale bars: 20 μm. (b) YO-PRO-1 fluorescence in Ca2+ free conditions (-Ca2+) was significantly different from control (“a” is equal to p < 0.0001; ANOVA) whereas uptake in the presence of four Cl− channel antagonists was not. Dye uptake was inhibited by stimulating cells in ECS containing 2 mM Ca2+ (+Ca2+; p < 0.0001; ANOVA). N = 4–7 separate experiments for all conditions. (c) Macrophages were pretreated with or without tannic acid (TA, 20 µM) or A01 (40 µM) for 30 mins and subsequently stimulated with or without ATP (2 mM) for 15 mins at 37°C. Supernatants were removed and replaced with dionized H20. Intracellular chloride of cell lysates was measured with MQAE (100 µM) and normalized to untreated controls (“a” denotes a significant increase from control (p<0.005). “b” denotes a significant decrease from ATP (p<0.005), n=9 separate experiments). (d) Ionomycin (1 µM) causes no measurable YO-PRO-1 uptake. (e) No significant difference in YO-PRO-1 fluorescence between untreated control macrophages and those treated with ionomycin, n = 4 separate experiments. “a” indicates significant difference from control.