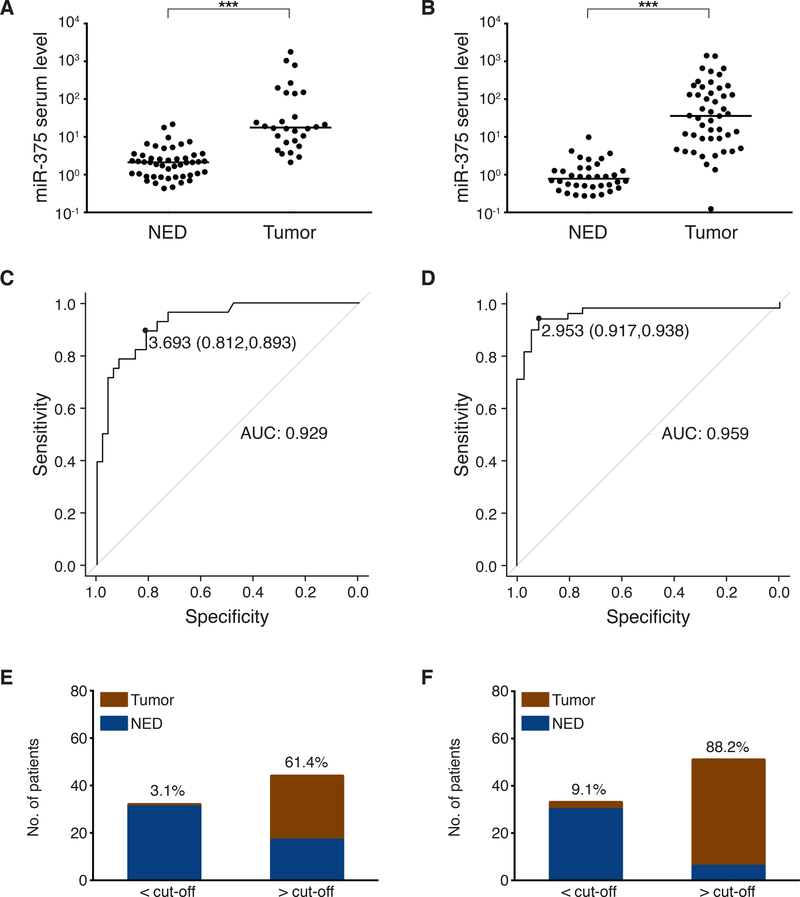
Fig. 4:
Circulating cell-free miR-375 in serum discriminates MCC patients with and without presence of disease: The prospective validation cohorts.
A, B: cf miR-375 in sera of MCC patients was determined by RT-qPCR in duplicate, and normalized to spiked-in cel-mir-39. Values were calculated relative to the serum of an MCC patient with no evidence of disease (Graz cohort) by the 2-ΔΔCq method. Results are depicted in Cleveland dot plots categorized in patients with no (NED) or with evidence of disease. C, D: Receiver operating characteristic (ROC) curves showing the sensitivity and specificity of miR-375 serum levels to discriminate tumor-bearing versus NED patients. The areas under the curve (AUC), optimal cut-off values and their sensitivity and specificity are given. E, F: The mean optimal miR-375 serum level cut-off was calculated from the optimal cut-off values of the retrospective discovery and validation cohorts as 2.42. Proportions of MCC patients of the prospective cohorts with (red) or without (blue) tumor burden below or above this mean optimal cut-off are depicted. Percentages of MCC patients with tumor burden within each group are given. A, C and E: Essen cohort, B, D and F: Melbourne cohort. Patients’ characteristics are given in S. Tab. 3–4. The horizontal line indicates the median, Mann-Whitney U test and pROC R were performed as described in Statistical analysis; p*<0.05, p**<0.005, p***<0.001.