Abstract
Replacement of exhausted, adsorptive media used to remove arsenic from drinking water accounts for approximately 80% of total operational and maintenance costs of this commonly used small system technology. Results of three full-scale system studies of an onsite media regeneration process (discussed in the first article of this two-part series) showed it to be effective in stripping arsenic and other contaminants from a granular ferric oxide (GFO) exhausted adsorptive media. This second article details the performance of the regenerated media to remove arsenic through multiple regeneration cycles and the approximate cost savings of regeneration over media replacement. Results indicated that media regeneration did not appear to have a major detrimental effect on the performance of the GFO media, and the regeneration cost was potentially less than the media replacement cost. Therefore, onsite regeneration offers small systems a possible alternative to media replacement when removing arsenic from drinking water using iron-based adsorptive media technology.
Keywords: adsorptive media, arsenic, drinking water treatment, regeneration
In the first article (Sorg et al. 2017) in this two-part series, results of three regeneration studies were presented. That study highlighted a three-step regeneration process of media backwash, caustic regeneration, and acid neutralization conditioning to strip arsenic and other contaminants from exhausted granular ferric oxide (GFO) media1 in the arsenic removal treatment system at the Twentynine Palms Water District (TPWD) in California.
This article, the second of two, presents performance data on the treatment system to remove arsenic following the three regeneration studies (detailed in Sorg et al. 2017) and the two subsequent studies conducted by TPWD. The authors also look at the estimated cost savings of each regeneration over media replacement.
MATERIALS AND METHODS
Arsenic treatment system and operation.
The TPWD arsenic removal system consists of two 5 ft-diameter adsorption vessels in parallel (tanks A and B); detailed design information for the system can be found in the first article of this series (Sorg et al. 2017). To extend the life of the adsorptive media, TPWD blends raw well water with the treated water; the blending ratio decreases with the increasing arsenic concentration of the system’s treated water. The US Environmental Protection Agency
(USEPA) has set a maximum contaminant level (MCL) for arsenic of 10 µg/L; the goal of the TPWD blending water process is to provide distribution water with an arsenic concentration of <8 µg/L. The decision of when to regenerate one tank of media was generally determined by the arsenic concentration of the finished blended water and not that of the adsorptive media effluent. Consequently, regeneration of the adsorptive media was carried out before the media had reached exhaustion (arsenic effluent at 10 µg/L).
Data and sample collection.
Because the first three regenerations (studies) were conducted with assistance from USEPA and its contractor (Battelle), monitoring of system performance after these three regenerations was supported by USEPA to collect more extensive performance data than what could be accomplished with only the TPWD resources.
Immediately following system startup after each of the three regeneration studies, an effluent grab sample was collected of the well water and the effluents from tanks A and B. The same three samples and a blended finished water sample were collected daily during the first week of operation and then weekly for weeks two, three, and four. After week four, this set of four samples was collected weekly along with a distribution water sample until the next regeneration. All samples were collected by TPWD personnel and sent to the Battelle chemistry laboratory in Columbus, Ohio, for the required analyses.
Following the third regeneration process in 2011, the TPWD regenerated one tank of media in 2013 and 2014. The treatment system was shut down in March 2015 when the finished water was found to have a hexavalent chromium (CrVI) level exceeding California’s revised MCL for CrVI of 0.010 mg/L or 10 µg/L (California State Legislature 2014). System performance monitoring by TPWD in 2013 and 2014 consisted of weekly samples of the raw water, effluents of tanks A and B, and the blended finish water. Analyses of these samples were limited to arsenic and were conducted at the Clinical Laboratory of San Bernardino, Calif.
Chemical analyses.
All water samples shipped to the Battelle chemistry laboratory were analyzed for arsenic, sulfate, uranium, phosphate, and silicate by inductively coupled plasma–mass spectrometry following the procedures detailed in a Battelle quality assurance/quality control plan produced by Battelle and approved by USEPA (Battelle 2004). The analytical method used for the arsenic analyses conducted by Clinical Laboratory was method SM 3113-B, AA Furnace (Standard Methods 2005).
RESULTS
The arsenic removal system, installed in September 2007, treated approximately 48 mil gal or 46,500 bed volumes (BVs) of water (23,250 BVs per tank) before the arsenic effluent reached 10 µg/L in October 2008. This run length represented about 40% of the vendor-guaranteed media life for the system of 116,000 BVs. The system was shut down until the first regeneration of the media in tank B took place during March 2009, and the media in tank A was replaced with new media.
Between 2009 and 2014, one tank of media was regenerated each year except during 2012 (Table 1). Tank B was regenerated three times and tank A twice. In most cases, media regeneration of a tank occurred when the arsenic concentration in the system effluent (blended water) approached 10 µg/L rather than when the effluent arsenic from at least one tank reached 10 µg/L. Therefore, the arsenic removal capacity (BV) of each individual tank of media was less than what could have been achieved had the media been used to an arsenic breakthrough of 10 µg /L. System performance during the five-year period 2009–2014 is shown in Figure 1.
Table 1.
Regeneration schedule and media performance at time of regeneration.
| Regeneration No. | Regeneration Tank | New Media Installed | Date of Regeneration | As Effluent – ug/L+ |
BV Treated Water x1000+ |
|||
|---|---|---|---|---|---|---|---|---|
| Tank A | Tank B | System Effluent* |
Tank A | Tank B | ||||
| 1 | Tank B - 1st | September, 2007 | March, 2009 | NA | NA | 10 | 45.6 | 45.6 |
| 2 | Tank B - 2st | September, 2007 | July, 2010 | 6.3 | 7.3 | 9.8 | 55.2 | 55.2 |
| 3 | Tank A - 1St | March, 2009 | April, 2011 | 9.3 | 2.8 | 8.0 | 81.8 | 27.6 |
| 4 | Tank B - 3rd | September, 2007 | June, 2013 | 5.4 | 8.2 | 8.5 | 45.3 | 70,8 |
| 5 | Tank A - 2nd | March, 2009 | September, 2014 | 10 | 5.7 | 9.1 | 85.1 | 42.2 |
At the time of regeneration.
Includes blended water.
Figure 1.
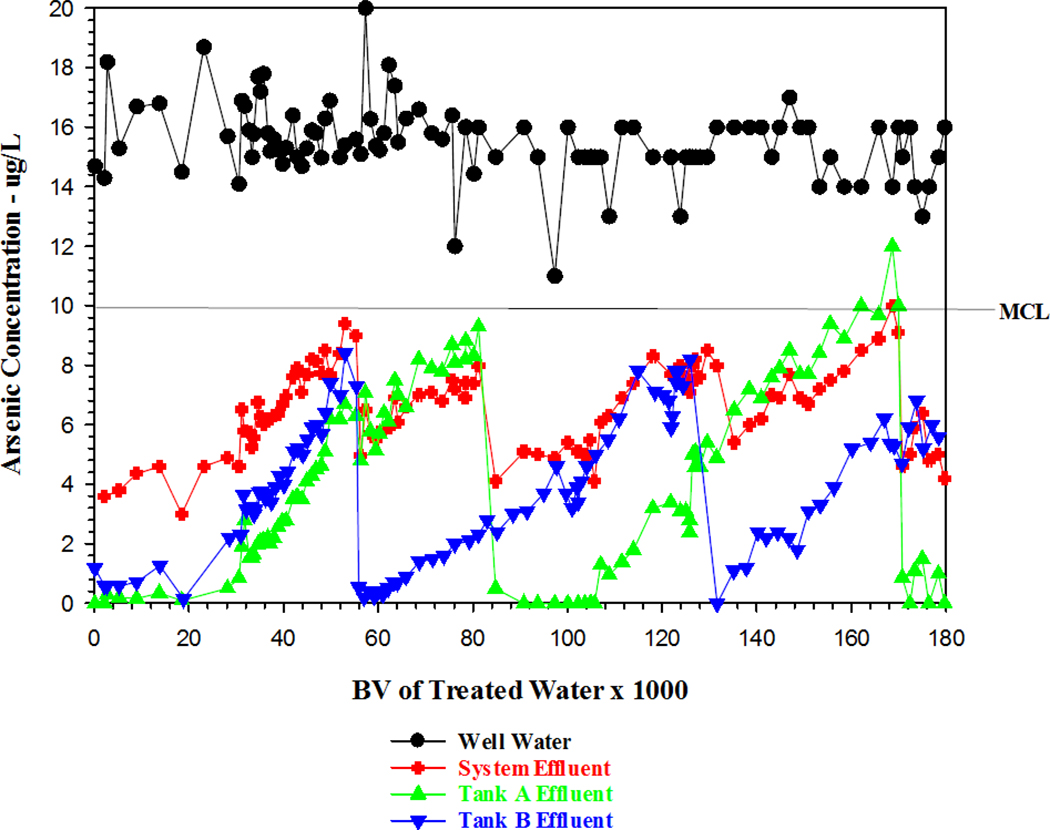
Performance of arsenic removal system
Tank B performance.
To make a reasonable comparison of the performance of the first regeneration process of the exhausted media of tank B to the performance of the new media of tank A, a very small amount of the new media of tank A was added to tank B to provide equal amounts of media in both tanks. Figure 1 compares the performance of the regenerated media (tank B) with the new media of tank A. As shown in the figure, at 55,000 BVs of treated water for each tank of media, the performance (effluent arsenic of 7.3 µg/L) of regenerated media (tank B) was similar to but slightly lower (effluent arsenic of 6.3 µg/L) than the new media (tank A). The slightly lower performance was expected because the amount of arsenic stripped from the exhausted media was in the range of 83–86% and not 100%.
At approximately 55,000 BVs (each tank), the arsenic concentration of finished, blended water was near 10 µg/L, indicating a need to regenerate one of the tanks of media. Although the effluent arsenic concentration from tank B was only 7.3 µg/L, the arsenic concentration from tank A (new media) was even lower (6.3 µg/L), which led to the decision to conduct a second regeneration of tank B media rather than the tank A media (Table 1). Even with an effluent arsenic concentration <10 µg/L in both tanks, the performances for both tanks of media were significantly higher than the performance (23,250 BVs each tank) of the original media. The poor performance of the original media was attributed to an oil film found in both tanks at the time of the first regeneration. The oil (which came from the submersible pumps) likely caused some fouling of the media. Following the caustic regeneration solution to clean up the media in tank B and the correction of the oil leak, the performance of the regenerated media improved.
Approximately three years after the second regeneration of the tank B media and around 72,800 BVs of treated water, a third regeneration of the tank B media was conducted. The effluent arsenic concentration of tank B was approximately 8 µg/L. Following this third regeneration, the effluent arsenic was very low at <1 µg/L, and the early stages of the breakthrough curve appeared to be similar to the performance of the media after the first two regenerations (Figure 2). The media performance results for the tank B media indicated that the three regenerations did not appear to have a major detrimental effect on the performance of this media.
Figure 2.
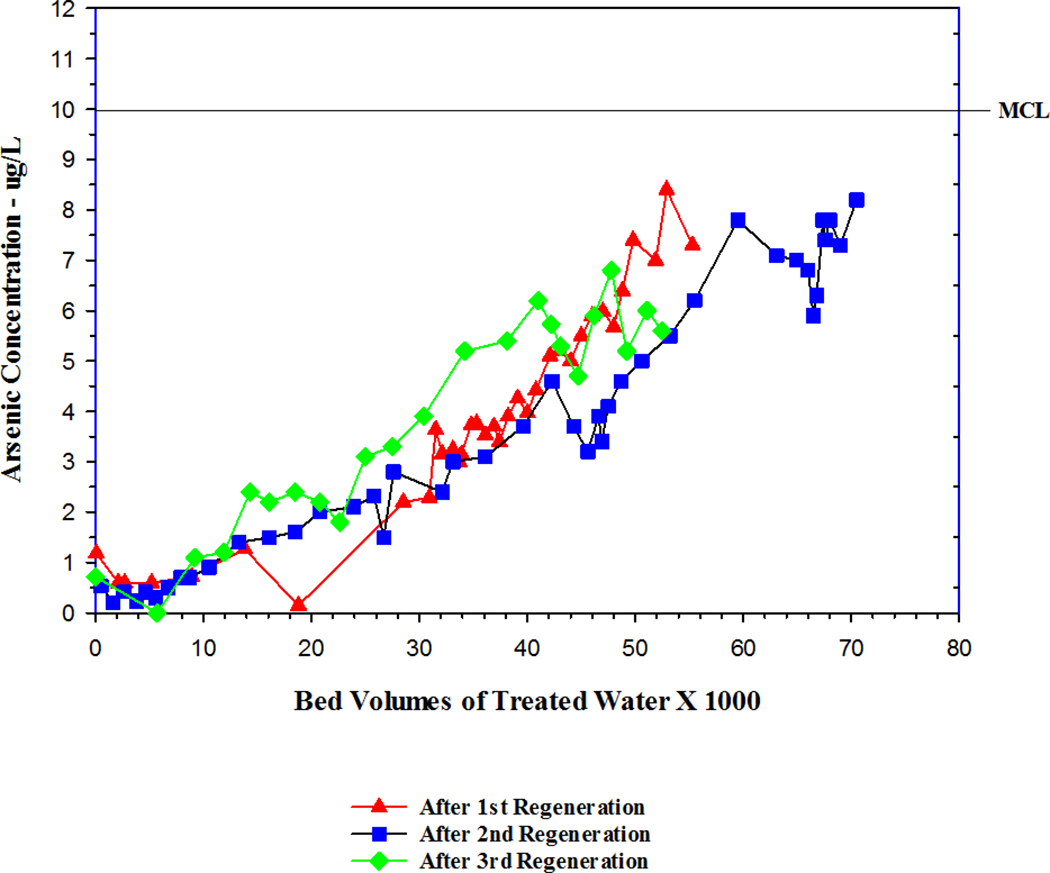
Performance of Tank B after each regeneration
Tank A performance.
Because the media in tank A was replaced with new media at the time of the first regeneration of tank B media, the performance of this new media was anticipated to be higher than the regenerated media. As shown in Figure 1, the new media had an effluent arsenic level of <1 µg/L for approximately 20,000 BVs, which was only slightly lower than the performance of the regenerated tank B media. At around 81,800 BVs, the effluent arsenic concentrations were 9.3 µg/L for tank A and 2.8 µg/L for tank B. The 81,800 BVs of treated water was actually higher than the vendor’s projected bed life for one tank of new media. The arsenic concentration of the finished blended water (20%) was 8.0 µg/L, and although the system could have continued operating for another few months, a decision was made to regenerate the tank A media for the first time. This regeneration took place in April 2011 before the ambient temperature in this desert location became too hot to conduct the field regeneration process.
The performance of the tank A regenerated media was very similar to the virgin media even in the very early stages when the arsenic level was <1 µg/L for the first 20,000 BVs of treated water. As shown in Figure 3, the breakthrough curve for the regenerated media paralleled the virgin media curve through 55,000 BVs, indicating that the initial regeneration process had little or no effect on media performance.
Figure 3.
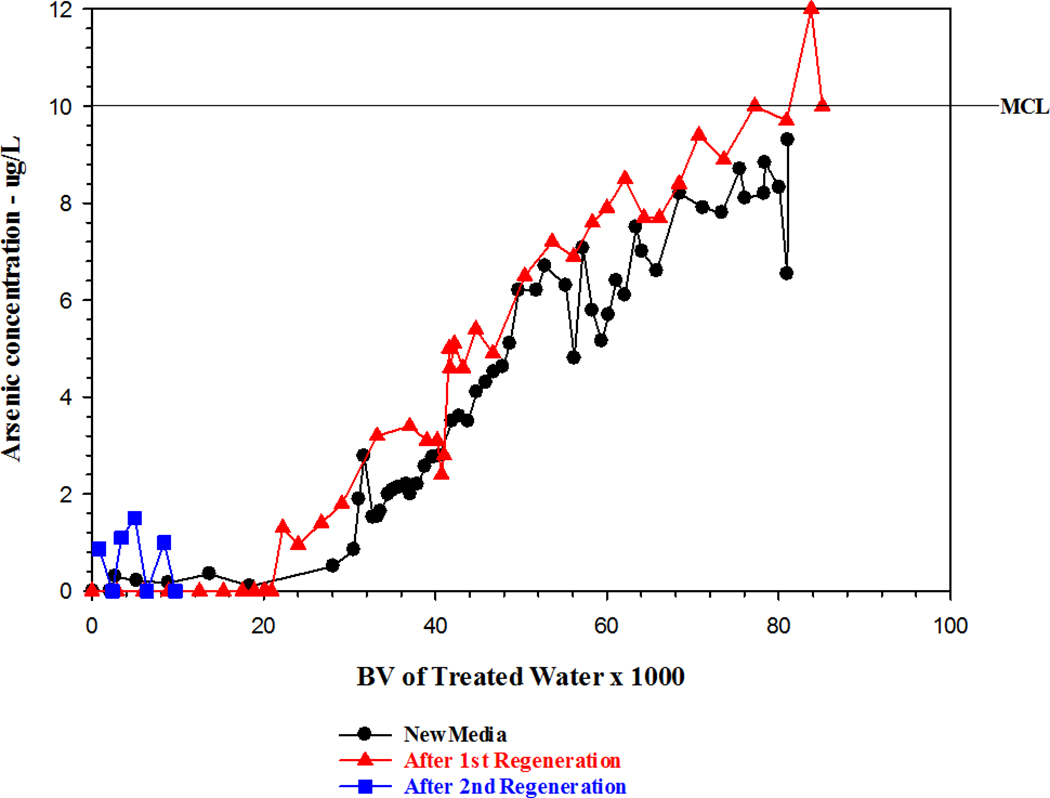
Performance of Tank A after each regeneration
On September 23–24, 2014, tank A was regenerated for the second time when the blended water concentration reached 9.1 µg/L and the media had treated 85,000 BVs (Figure 3). The effluent arsenic concentrations of tanks A and B were 10 and 5.7 µg/L, respectively. After regeneration, when the system was placed back in service (Oct. 20, 2014), the effluent arsenic of tank A was <2 µg/L for the first 9,000 BVs. These results indicated that the second regeneration had a very minor effect on system performance during the early life of the media. In March 2015 the treatment system was shut down because the finished water concentration of CrVI exceeded California’s revised MCL of 10 µg/L.
Regeneration process costs.
Between March 2009 and September 2014, the TPWD regenerated a tank of media five times: three regenerations of tank B media and two of tank A media (Table 1). The regeneration processing costs included the chemicals (caustic and acid), labor, and wastewater disposal. The processing costs presented in Table 2 do not include the initial capital costs of plumbing modifications, caustic solution and wastewater storage tanks, chemical feed pumps, pH meters and supplies, and a portable arsenic test kit, or any monitoring costs of the research studies. The costs of the system changes amounted to approximately $3,000 and are not included in the regeneration costs discussed in the following sections. The percentage of the $3,000 assigned to each regeneration depended on the total number of regenerations conducted and decreased with each regeneration completed.
Table 2.
Cost and savings of each individual regeneration process.
| Regeneration Process | Date of Regeneration | Regeneration Process – Cost ($) | ||||
|---|---|---|---|---|---|---|
| Chemicals | Subcontractor | Waste Disposal | Total Cost | Savings* | ||
| Tank B - 1st | March, 2009 | $158 | $4,641 | $10,040 | $14,839 | $5,686 |
| Tank B - 2nd | July, 2010 | $284 | -- | $4,618 | $4,902 | $15,623 |
| Tank A – 1st | April, 2011 | $368 | -- | $5,343 | $5,711 | 14,814 |
| Tank B - 3rd | June, 2013 | $250 | -- | $10,282 | $10,532 | $9,983 |
Based on estimated cost of media replacement of $20,525.
As shown in Table 2, the cost of the first regeneration process (tank B) was $14,839 and consisted of $158 for chemicals, $4,641 for subcontractor help, and $10,040 for transportation and disposal of the wastewater at a chemical waste disposal facility. For this first regenera-tion process (tank B), the quantity of wastewater produced and the cost of its disposal was not a major concern; as a result, the wastewater transportation and disposal cost represented the highest cost component (68%) of the total cost. The cost of replacing a tank of media (69 ft3) was estimated at $20,525 during the study period. Consequently, even with a fairly high wastewater disposal cost and the subcontractor expense, the cost of the regeneration process (not including any costs associated with system modifications) was $5,686 less than the cost of media replacement.
Having determined that the regeneration process was effective in stripping the arsenic from the media during the first regeneration process, TPWD staff turned their attention to refining the regeneration process to reduce the quantity of wastewater during the second regeneration of tank B (Sorg et al. 2017). By reducing the quantity of the wastewater, processing the wastewater on site, and locating a lower-cost wastewater disposal company, TPWD was able to lower the regeneration cost to slightly less than $5,000, with offsite wastewater disposal again the major cost. Because only TPWD personnel were used to conduct the second regeneration process and all subsequent regenerations, no out-of-pocket cost for subcontractor assistance was required. The $5,000 regeneration cost does not include any costs associated with system modifications or TPDW personnel costs. These changes resulted in a cost savings of $15,623, which was approximately $10,000 less than the cost of the first regeneration process (Table 2).
The third regeneration process (the first regeneration of tank A media) closely followed the procedures of the second regeneration process. The cost of the process was approximately $6,000, or about one-fourth the cost of media changeout. The cost data for the first three regenerations clearly indicate that the cost of the chemicals was insignificant compared with the cost of wastewater disposal, which accounted for around 95% of the total cost.
Wastewater disposal.
As discussed previously, the total regeneration process cost is almost entirely a function of wastewater disposal. Given that TPWD was unable to dispose of the high-arsenic concentration wastewater (considered a hazardous waste) at a district-owned facility, the only option available was offsite disposal at a chemical waste disposal plant. With the results of the first regeneration study clearly demonstrating some cost benefits of regeneration, the second regeneration study that followed a year later focused on lowering the wastewater disposal cost.
Two modifications were made to the second regeneration process to reduce the quantity and the characteristic of the wastewater. First, the total quantity of wastewater produced was reduced by approximately 1,700 gal by eliminating the water rinse step that followed the caustic regeneration step (Sorg et al. 2017). This change was also carried out during all subsequent regenerations.
The second modification to the second regeneration process involved the addition of ferric chloride (FeCl3) to both the spent caustic solution and the acid neutralization wastewater; this modification reduced the arsenic concentration of both solutions to a level that would allow the liquid fraction of both wastes to be recycled with the system’s influent raw water. To reduce the arsenic concentration of the 800 gal of spent caustic regenerant and 3,000 gal of the neutralization wastewater (held in separate holding tanks), an FeCl3 (40%) solution was added to each storage tank, with the quantity based on a target Fe-to-As (iron-to-arsenic) ratio of around 30:1. The pH of each wastewater was also lowered to about 6.5 with sulfuric acid. Results of the wastewater parameters of the onsite processing are provided in Table 3.
Table 3.
Summary of on-site wastewater processing parameters
| Parameter | Unit | Spent Caustic | Neutralization Water |
|---|---|---|---|
| Estimated Volume | gal | 800 | 3,000 |
| Initial pH (Before FeCl3 Treatment) | S.U. | 13.0 | 12.1 |
| Initial Arsenic Concentration - ICP-MS* | mg/L | 286 | 30.9 |
| Amount of 40% FeCl3 Addition | gal | 55 | 18 |
| Fe:As Ratio | 47:1 | 38:1 | |
| Final pH after FeCl3 Addition/pH Adjustment | S.U. | 6.0–6.2 | ~6.5 |
| Final Arsenic Concentration – Lab ICP-MS | µg/L | 82.8 | 29.4 |
ICP-MS = inductively couple plasma-mass spectrometry
By adding 55 gal of liquid FeCl3 to the 800 gal of spent caustic solution and adjusting the pH to around 6.5, the arsenic concentration of the caustic solution was lowered from 285,250 µg/L to 82.8 µg/L, for a reduction of 99.97% (Table 4). The arsenic concentration of a portion (575 gal) of the liquid fraction was found to be further reduced to 35 µg/L by passing it through a bag filter followed by an approximately 1 ft3 cartridge filter containing an iron-based media2 (Table 4). The media is somewhat similar to the GFO used in this research and was on hand from another Battelle arsenic demonstration project.
Table 4.
Characteristics of processed recycle wastewaters.
| Parameter | Unit | Wastewater Before FeCL3 Treatment | Supernatant after FeCl3 Treatment | % Removal by FeCl3 Treatment | Supernatant Processed by ARM 200 | % Removal by ARM 200 |
|---|---|---|---|---|---|---|
| Spent Caustic (800 gal) | ||||||
| Date | - | 07/16/10 | 07/17/10 | - | 07/23/10 | - |
| pH | S.U. | 13 | 6.0–6.2 | - | 7.2 | - |
| As (total) | µg/L | 285,250 | 82.8 | 99.97% | 35.1 | 57.6% |
| As (soluble) | µg/L | 286,890 | 40.7 | 99.99% | 36.9 | 9.4% |
| Fe (total) | µg/L | <250 | 1,155 | 0.0% | 32 | 97.3% |
| Fe (soluble) | µg/L | <250 | <250 | 0.0% | <25.0 | 90.0% |
| P (total) | µg/L | 46,093 | 253 | 99.5% | 43.8 | 82.7% |
| P (soluble) | µg/L | 45,927 | <50 | 99.9% | 17.4 | 30.3% |
| Si (total) | µg/L | 425,337 | 11,367 | 97.3% | 746 | 93.4% |
| Si (soluble) | µg/L | 439,387 | 11,242 | 97.4% | 574 | 94.9% |
| U (total) | µg/L | 15,074 | < 1.0 | 99.997% | 0.3 | 39.6% |
| U (soluble) | µg/L | 15,266 | < 1.0 | 99.997% | 0.2 | 50.2% |
| Neutralization/Rinse Wastewater ( 3000 gal ) | ||||||
| Date | - | 07/16/10 | 08/09/10 | - | 08/30/10 | - |
| pH | S.U. | 12.1 | 6.5 | - | 7.5 | - |
| As (total) | µg/L | 30,881 | 29.4 | 99.90% | 12.6 | 57.2% |
| As (soluble) | µg/L | 30,919 | 14.3 | 99.95% | 9.1 | 36.1% |
| Fe (total) | µg/L | <250 | 423 | 0.0% | 160 | 62.2% |
| Fe (soluble) | µg/L | <250 | <25 | 90.0% | <25.0 | 0.0% |
| P (total) | µg/L | 4,215 | <5.0 | 99.9% | 42.3 | 0.0% |
| P (soluble) | µg/L | 4,210 | <5.0 | 99.9% | 43.0 | 0.0% |
| Si (total) | µg/L | 91,850 | 21,299 | 76.8% | 3,525 | 83.5% |
| Si (soluble) | µg/L | 89,254 | 20,798 | 76.7% | 3,027 | 85.4% |
| U (total) | µg/L | 1,458 | 2.2 | 99.97% | 0.8 | 65.7% |
| U (soluble) | µg/L | 1,455 | 0.7 | 99.97% | 0.4 | 36.5% |
A total of 18 gal of FeCl3 was added to the 3,000-gal neutralization wastewater, and this solution was adjusted to pH 6.5 with concentrated sulfuric acid. The addition of FeCl3 and acid reduced the arsenic concentration from 30,881 µg/L to 29.4 µg/L, for a 99.9% reduction. Approximately 300 gal of liquid fraction was passed through the bag filter and the alumina-based media, resulting in a further reduction of the arsenic concentration to 12.6 µg/L.
In addition to removing a very high percentage of the arsenic, the FeCl3 and the iron-based media also achieved high removal of phosphorus, silicon, and uranium (Table 4). The results of these tests were provided to the CDPH along with a request that the TPWD be allowed to recycle the liquid fraction of the processed wastewater to the head of the treatment plant using either a 20 or 5 gpm pump. Because the treatment system operates at 296 gpm and has a bypass flow as high as 105 gpm, use of the 20 gpm pump would result in a 16× dilution of the recycled streams, and use of the 5 gpm pump would result in a 60× dilution of the recycled streams. For future operations, the TPWD proposed to combine the two solutions before FeCl3 addition and pH adjustment.
After a review of the process wastewater test results and TPWD’s request for approval of the recycle process, CDPH raised several questions about the characteristics of the recycled stream and requested additional information beyond that provided by TPWD. The state also raised concerns about the effect of the recycled regenerant on the GFO media and would consider approval only if extensive water quality tests were conducted on the recycled stream each time the process was carried out. Because of the effort required and subsequent cost to respond to these issues, TPWD chose not to pursue the use of this recycling process and continued to haul the wastewater off site for disposal.
The changes in the characteristics of the wastes to a liquid and iron solids did provide some cost benefit with regard to the total disposal cost. Because the arsenic concentration of the liquid fractions was significantly lower, the waste-disposal-company cost quote was based on the quantity of liquid waste and solids (as shown in Table 5). These cost data indicated that additional cost savings could be achieved if the liquid fraction of the processed regenerant wastewater could be recycled to the front of the treatment system, leaving only the iron solids for disposal. Moreover, drying the solids would also reduce the quantity of solids, and these dried solids would likely pass the toxicity characteristic leaching procedure test, allowing the solids to be disposed at a sanitary landfill at an even lower cost. Although TPWD did not pursue these steps, these waste reduction actions could be considered by systems that may be interested in onsite regeneration of exhausted media.
Table 5.
Regenerant wastes disposal cost quotation.
| Item | Cost* |
|---|---|
| Liquid/As | $0.45/gal |
| Solids | 0.0659/gal (solids at 9–14%) |
| Transportation | $85/hr |
| Truck Washout | $125/Truck |
| Fuel Charge | $178 |
May, 2011
CONCLUSIONS
The regeneration studies conducted on the TPWD adsorptive media (GFO) arsenic removal system yielded the following conclusions regarding the performance of the regenerated media and the cost savings of regeneration over media replacement.
The arsenic removal capability of a regenerated GFO media is similar to that of virgin media, although the life of the regenerated media will be slightly less.
Multiple regenerations (in this case, three) of an exhausted GFO media did not appear to have a significant detrimental effect on the performance of this media.
Cost savings can be achieved by regenerating an exhausted GFO media over replacing the media with new media.
If the water utility does not have an onsite option for disposing of the regenerant wastewater, the most significant cost element of the regeneration process is media disposal, which could amount to 95% of the total regeneration costs.
Adding FeCl3 to the regenerant wastewaters and adjusting their pH to around 6.5 can result in the removal of 99% or more of the arsenic from the wastewaters. Because of the low arsenic concentration of the liquid fraction of the processed wastewaters, the liquid fraction has the potential to be recycled to the front of the treatment system.
Recycle of the liquid fraction of a processed wastewater to the front of the treatment plant could result in a regeneration cost that is only 10–15% of the cost of replacing the exhausted media with new media.
ACKNOWLEDGMENT
The authors thank the staff of the Battelle chemical laboratory in Columbus, Ohio, who were responsible for the analyses of the water and media sample. The authors also acknowledge efforts of the Twentynine Palms (Calif.) Water District regeneration team involved in all of the regeneration processes and their help with data collection in the regeneration studies.
Footnotes
Publisher's Disclaimer: DISCLAIMER
The USEPA, through its Office of Research and Development, funded, managed, and collaborated in the research described here. This article has been subjected to the agency’s administrative review and has been approved for external publication. Any opinions expressed in this article are those of the authors and do not necessarily reflect the views of USEPA; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
ENDNOTES
1Bayoxide® E33, Severn Trent Services, Fort Washington, Pa.
2ARM® 200, Englehard, Iselin, N.J.
REFERENCES
- Battelle, 2004. Quality Assurance Project Plan (QAPP) for Evaluation of Arsenic Removal Technology US Environmental Protection Agency, Cincinnati. [Google Scholar]
- California State Legislature, 2014. SB-385: Primary Drinking Water Standards Hexavalent Chromium; Sacramento, Calif. [Google Scholar]
- Sorg TJ; Chen ASC; Wang L; & Kolisz R, 2017. Regenerating an Arsenic Removal Iron-Based Adsorptive Media System, Part 1: The Regeneration Process. Journal AWWA , 109:5:13 10.5942/jawwa.2017.109.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 2005. (21st ed.). APHA, AWWA, and WEF, Washington. [Google Scholar]


