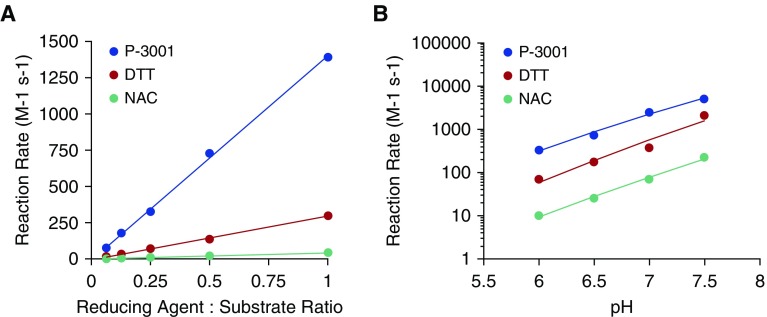
Figure 1.
The reduction potential of P3001 exceeds that of dithiothreitol and N-acetylcysteine. Spectrophotometric studies monitored formation of reduced 5′,5′-dithio-bis(2-nitrobenzoic acid) over time. Reaction rates, generated using second-order kinetics, for each compound were compared as a (A) function of reducing agent concentration at pH 6.5 or (B) over a relevant pH range. The x-axis reflects the pseudo–second-order kinetics of the reducing reaction rates per molar per second. DTT = dithiothreitol; NAC = N-acetylcysteine.