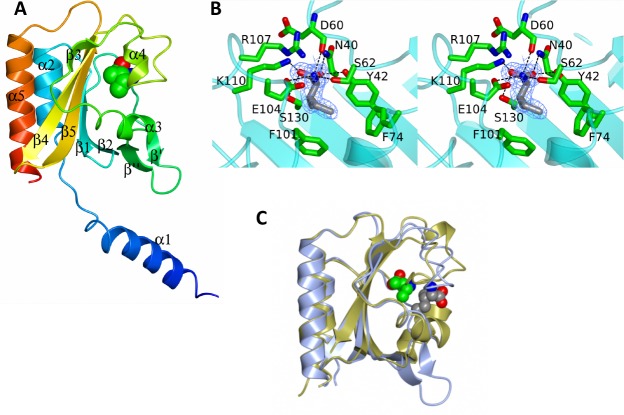
Fig 5. Structure of the GAF domain of C. difficile CodY.
A. Ribbon tracing of chain A color ramped from the N-terminus (blue) to the C-terminus (red). The secondary structure elements are labeled. The isoleucine cofactor is shown as spheres and colored by atom: carbon, green; nitrogen, blue; oxygen, red. B. Stereo view of the ligand binding site with the isoleucine effector shown in cylinder format and with carbon atoms colored grey. 2Fo-Fc electron density associated with the effector in the refined structure is contoured at 1σ and shown in light blue. Surrounding protein residues are shown in cylinder format with their carbon atoms colored in green. C. Comparison of the GAF domains of CodY from C. difficile and B. subtilis shown as blue and gold ribbons respectively with the isoleucine ligands shown as spheres with grey and green carbon atoms respectively. The different positions and orientations of the effector molecules are apparent.