Abstract
Chordoma is a slowly growing and invasive bone tumor with a tendency to metastasize locally in advanced stages. It is essential to discover new therapeutics that target genes involved in the metastasis of chordoma. Epithelial-mesenchymal transition (EMT) might robustly influence the metastasis of a tumor bulk. To our knowledge, this is the first time to show that EMT might have a role in chordoma metastasis. In this study, we aim to investigate the possible role of Twist, a key player transcription factor of EMT, in chordoma metastasis. The TWIST gene was silenced by short hairpins in chordoma cell line MUG-Chor1 and effects on metastasis were investigated by wound healing/gap closure and invasion assays. Twist-silenced MUG-Chor1 cells were found to be less migratory and less invasive when compared to the negative control. This study indicates that Twist might have a role in metastatic chordoma cells.
Keywords: Cancer, epithelial-mesenchymal transition, chordoma, twist
1. Introduction
Chordoma, an aggressive and locally invasive bone neoplasm, accounts for 1%–4% of all primary bone tumors, affecting 0.1/100,000 people in a year. It is considered to be the second most malignant tumor of the spine (McMaster et al., 2001) . Although it is known as a lowgrade tumor, chordoma tends to relapse and metastasize locally (Yang et al., 2009) , being nonselective to tissues and organs such as the lungs, bones, liver, soft tissues, lymph nodes, and skin. Rare events of metastasis into the subcutaneous tissue, muscle, heart, pleura, spleen, kidney, bladder, pancreas, and brain have also been reported (Bergh et al., 2000; McPherson et al., 2006; Vergara et al., 2008) . Various factors like local relapse, increased tumor size, necrosis, and exposure to high dose radiation may indicate the metastatic prognosis of chordoma (Chambers and Schwinn, 1979; Markwalder et al., 1979; McPherson et al., 2006) . A study done by Bergh et al. showed that local recurrence is related significantly with a greater risk of metastasis and tumor-related death (Bergh et al., 2000) . In addition, there is not a clear relationship between age, sex, and origin of the tumor with metastasis (McPherson et al., 2006) .
Metastasis is a combination of events that occur during carcinogenesis (Eccles and Welch, 2007) . Cancer cells evolve into a mesenchymal state from an epithelial state to become aggressive and metastatic and this process is regulated by epithelial to mesenchymal transition (EMT) (Thiery, 2003; Huber et al., 2005) . EMT is a process where cells lose their polarity and become more motile. As reported extensively by several studies, E-cadherin expression is downregulated as an initial step of EMT (Thiery, 2002; Zhou et al., 2004; Bates and Mercurio, 2005) . E-cadherin is the key component of cell-cell adhesion junctions and is necessary for the formation of epithelia in the embryo. It eventually maintains epithelial homeostasis in adults. During development and carcinogenesis, E-cadherin is lost at sites of EMT. Eventually, tumor cells become invasive in vitro and loss of E-cadherin causes the transition of adenoma to carcinoma in in vivo models (Thiery, 2002). One of the factors that aefct the downregulation of E-CADHERIN expression is the transcription factor TWIST. Studies done by Yang et al. and Cheng et al. demonstrated that Twist is primarily overexpressed in metastatic cancers (Yang et al., 2006; Cheng et al., 2008) . When it is downregulated, tumor cells evade intravasation, a process in which metastatic cells enter into blood circulation to travel to other sites of the body to find new locations (Yang et al., 2006) . In addition to its role in organogenesis, recent studies show that Twist is an essential player in triggering metastasis in numerous kinds of tumors like breast cancer, hepatocellular carcinoma (HCC), prostate cancer, and gastric cancer (Chen and Behringer, 1995; Yang et al., 2004; Matsuo et al., 2009; Yang et al., 2007) .
A recent study showed that, other than Brachyury, another embryonic transcription factor like Twist might have a role in the tumorigenesis of chordoma (Vanderheijden, 2014) . Therefore, we conducted the present study to investigate the possible role of Twist in cell migration of chordoma cells and its role in metastasis.
2. Materials and methods
2.1. Cell cultures
The MUG-Chor1 cell line was provided by the Chordoma Foundation (Durham, NC, USA). Cells were cultured as described by Rinner et al. (2012) in a special culture medium of IMDM/RPMI (4:1) supplemented with 10% FBS and 1% antibiotics (GIBCO, USA) in flasks covered with 1% gelatin. Cells were incubated in a humidified chamber at 37 °C in 5% CO2.
2.2. Viral particles and gene silencing
MUG-Chor1 cells were transfected with short hairpin lentiviral particles of TWIST (sc-38604-V, Santa Cruz Biotechnology, USA), Control-A shRNA (sc-108080, Santa Cruz Biotechnology), and copGFP Control (sc108084, Santa Cruz Biotechnology) as instructed by the manufacturer. In brief, cells were seeded to 12-well culture plates at 80% conuflency and incubated with culture medium containing 5 µg/m: polybrene (sc-134220, Santa Cruz Biotechnology) for 6 h. Lentiviral particles, at preoptimized MOI values, were added to the medium and cells infected with the viral particles were incubated for 24 h. The culture medium was renewed with complete medium containing 1 µg/mL puromycin dihydrochloride (sc-108071, Sigma Aldrich, USA). Puromycin-resistant colonies were selected over 2 months due to slow growth of MUG-Chor1 cells upon viral infection (nearly 5 days of doubling time). Over 2 months of selection period a stable cell line was generated and verified by gene and protein quantifications.
2.3. Gene expression/protein quantification
The changes in gene expression were measured with qPCR. Cells were collected, total RNA was isolated by the NucleoSpin RNA isolation kit (Macherey Nagel, USA), and cDNAs were synthesized by the QuantiTect Reverse Transcription Kit (205313, QIAGEN, USA) as instructed by the manufacturers. Real-time qPCR was conducted with TaqMan Fast Universal PCR Master Mix (4352042, Thermo Scientific, USA) with the TaqMan probe against TWIST (Hs01675818_s1, Thermo Scientific). Normalization was done against GAPDH and relative foldchanges were calculated by the 2–ΔΔCt method. Results were analyzed with the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA) and CFX Manager Software (V. 3.0, Bio-Rad).
Twist suppression was also confirmed by protein analysis. Brieyfl, the cell pellet was resuspended in RIPA buffer (R0278, Sigma, USA) containing protease and phosphatase inhibitor (78430, Thermo Scientific) at a volume according to the size of the pellet. Protein quantitation was done by BCA Bradford assay (23227, Thermo Scientific). Twist protein was measured with ELISA assay (E3531Hu, BT Laboratories, China) as described in the manufacturer’s protocol. Absorbance was measured with an ELx800 96-well microplate reader (MTX Lab Systems, USA) and results were normalized to GAPDH.
The effect of Twist silencing on proliferation was observed by comparison of cell counts over 5 days. To do this, Mug-shCtrl cells and Mug-shTwist cells were seeded onto 48-well plates as 10 × 103 cells/well. The quantity of formazan, which is considered to be directly proportional to the number of viable cells, was measured by recording changes in absorbance at 490 nm using a plate-reading spectrophotometer for 5 consecutive days. Calculations were made accordingly.
2.4. Scratch assay
MUG-Chor1 cells, silenced against TWIST (shTwist) and a negative control (shCtrl, i.e. no gene knockdown), were seeded into 6-well plates at 1× 105 cells/well. Aeftr attachment, cells were scraped with a 100-µL sterile pipette tip for wound induction. Detached cells were removed and the serum concentration was reduced to 2%. Cells were monitored and images were taken from three predetermined sites of the scratch under an inverted microscope (ZEISS Axio Vert.A1, Zeiss, Germany) every day until the gap of the control cells was completely covered. Scratch images were evaluated by the ImageJ Mitobo plugin (ImageJ Software, NIH, USA). The scratch assay was repeated three times and average values were calculated.
2.5. Gap closure assay
Gap closure assay was performed using the Radius 24well cell migration assay (CBA-125, Cell Biolabs, USA) according to the manufacturer’s protocol. Brieyfl, culture wells were treated with pretreatment solution. Then cells were seeded and incubated overnight for attachment. The next day, culture medium was removed and wells were treated with medium containing gel removal solution. Wells were rinsed with fresh culture medium twice and cultured with 2% FBS containing culture medium. Images of three wells for each group were taken under an inverted microscope every day until the gap of the negative control group was covered completely. Gap closure averages were calculated from the results of three independent experiment sets.
2.6. Invasion assay
A combination of two different invasion assay kits was used to determine the invasion capabilities of chordoma cells upon Twist silencing. First cells were seeded onto Matrigel-coated inserts (354480, Corning BioCoat Matrigel Invasion Chamber, Corning, USA) and the protocol was used as suggested by the manufacturer. For an internal control, control inserts (354578, Corning, USA, not provided by the kit) were used. Matrigel-coated inserts and control inserts were placed in a 24-well plate. Serum-free medium (IMDM/RPMI 4:1, 1% PSA) was added to each insert and the plate was incubated at room temperature for 1 h. Cells, in preoptimized numbers, were seeded into insert wells with serum-free medium and complete medium was added to the lower chamber. The plate was incubated for 48 h. Following 2 days of incubation, the interior of the insert was cleaned with a wet cotton swab and the remaining cells were stained with Giemsa stain (similar to Dif Quick stain to enhance the visibility of the cells, which is not provided but required in the protocol). Images from vfie different spots were taken under an invert microscope. Invasion values (Invasive Index) were calculated by comparing the invaded cells of the Matrigel insert to the control insert (without Matrigel). Compared to the control insert, 7% of shTwist cells passed through the Matrigel and 35% of shControl cells passed through the Matrigel.
2.7. Statistical analysis and image processing
Experiments were completed in three biological replicates with three technical triplicates in each experiment and statistical analysis was done by one-way ANOVA with Dunnett’s posttest using GraphPad Prism 5 (GraphPad Software, USA). A single asterisk indicates that P < 0.05. Graphics were exported from GraphPad Prism’s graphic generator. Cell counting, gap closure, and scratch assay analyses were done using ImageJ (National Institute of Health, USA) and the ImageJ Mitobo plugin.
3. Results
3.1. Gene expression analysis and protein quantitation assays
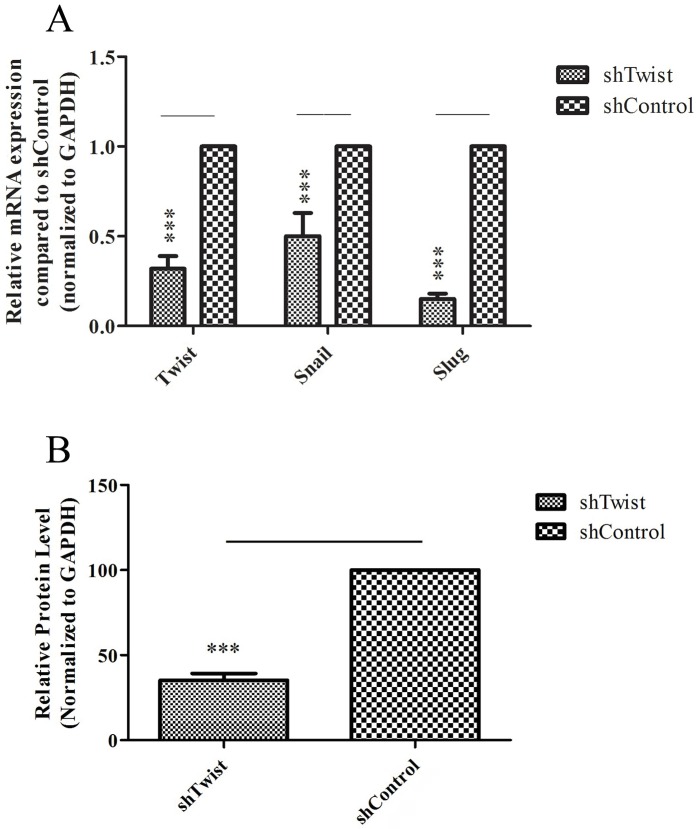
Following transfection with viral particles, gene expression and protein analyses were applied to confirm the silencing for the TWIST gene. As illustrated in Figure 1A, TWIST expression was knocked down in virus-infected MUGChor1 cells represented as Mug-shTwist compared to unsilenced (Mug-shCtrl). As for protein analysis (Figure 1B), it was found that Twist protein amount was decreased to 35% in Mug-shTwist cells compared to Mug-shCtrl cells. The effect of Twist silencing on proliferation rate was measured and no significant changes were obtained when compared to the Mug-shCtrl cells (Supplementary Figure 1).
Figure 1.
A) Relative Twist, Snail, and Slug gene expression levels were normalized to GAPDH. Triple asterisks indicate P < 0.001. B) Relative Twist protein levels were normalized to GAPDH. Triple asterisks indicate P < 0.001. Error bars represent standard deviation.
3.2. Wound healing assay (scratch assay)
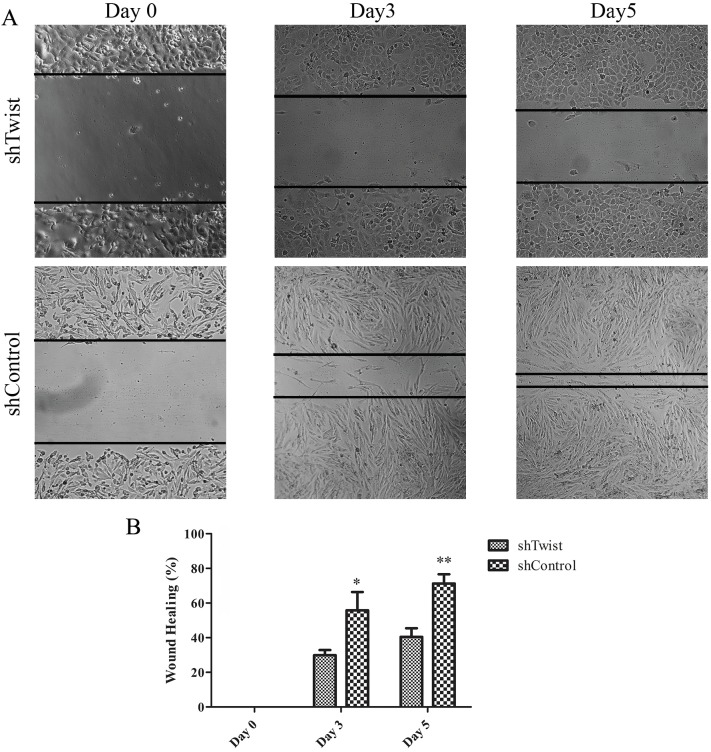
Migratory ability of Twist-silenced cells was analyzed through scratch assay. As can be seen in Figure 2A, the control group closed the scratched area at the end of 5 days while it was not closed thoroughly in Twist-silenced MUGChor1 cells. The percentage closure rate is represented in Figure 2B. Around 40% of the area was covered by shTwist cells while the control cells were covered by almost 90%.
Figure 2.
A)Wound healing assay. B) Graphical representation of wound healing percentages. Single asterisk indicates P < 0.05 and double asterisks indicate P < 0.01. Error bars represent standard deviation.
3.3. Gap closure assay
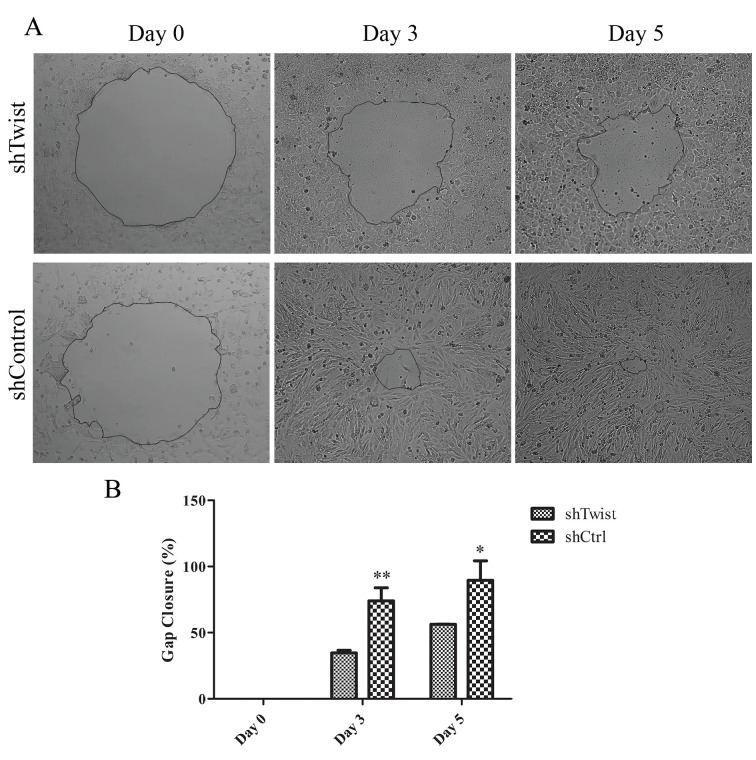
To observe the migratory ability of Twist-silenced MUGChor1 cells, gap closure assay was also applied. Images in Figure 3A illustrate that control MUG-Chor1 cells almost closed the gap, unlike Twist-silenced MUG-Chor1 cells, at the end of the 5th day. Figure 3B shows that 50% of the area was closed by the shTwist cells while 90% of the area was covered by control cells.
Figure 3.
A) Gap closure assay. B) Graphical representation of gap closure assay. Single asterisk indicates P < 0.05 and double asterisks indicate P < 0.01. Error bars represent standard deviation.
3.4. Invasion assay
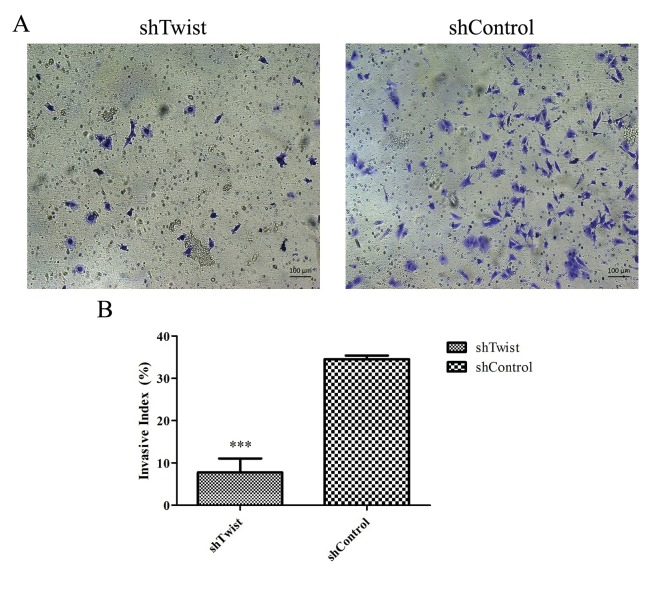
To indicate the effect of silencing on invasiveness of MUGChor1 cells, invasion assay was carried out and the results in Figure 4A show that control group invaded the Matrigel membrane more than Twist-silenced cells under the same conditions. The percentage rates of invasion of both cell types are indicated in Figure 4B. Only 7.85% of Twistsilenced cells passed through the Matrigel membrane while 34.5% of the control group invaded.
Figure 4.
A) Comparison of invasiveness between Twist-silenced (left) and control (right) MUG-Chor1 cells (10× magnification). B) Graphical representation of the invasive index (invasiveness compared to the control insert) of shTwist and shControl cells. Single asterisk indicates P < 0.05 and double asterisks indicate P < 0.01. Error bars represent standard deviation.
4. Discussion
Most cancer-related deaths result from metastasis due to the spread of cancerous cells to other parts of the body, allowing the formation of new cancer sites. Like in all other cancer types, metastasis is a challenge in chordoma patients. In our study, a chordoma cell line, MUG-Chor1, established by Rinner et al. (2012) , was used as a model to investigate possible target genes on the metastatic pathway. Known for its association with metastasis (Yang et al., 2004) , Twist, as the key regulator of EMT, was targeted to decrease the migration and invasion capability of chordoma.
Silencing of EMT was carried out by knocking down TWIST. Premade shRNA lentiviral particles were used to knock down TWIST, and confirmations of silencing were performed by gene expression analysis and protein quantification. Twist-silenced MUG-Chor1 cells were found to be less migratory, as demonstrated by wound healing, gap closure, and invasion assays, compared to control cells. These results show that silencing of the TWIST gene also results in reducing not only migration but also invasion, as proposed in many studies (Kwok et al., 2005; Vesuna et al., 2008; Croset et al., 2014; Liu et al., 2014) . Despite its slow growing behavior, chordoma is considered one of the malignant tumors of bone. Twist expression might be one of the factors to cause this.
Depending on the type of chordoma cases, the expressions of EMT markers vary. A case study done by Karamchandani et al. (2013) revealed that an aggressive type of chordoma strongly expresses the markers of EMT including vimentin, N-cadherin, Slug, and Twist. The highly proliferative features of this type of tumor are associated with the presence of expression of these markers. In our study we also knocked down the expression of other EMT markers including SNAIL and SLUG (Yang et al., 2009) ; however, the effect on migration capacity did not change significantly (data not shown) as happened in TWIST.
Some studies suggested that knockdown of TWIST inhibits cancer progression and metastasis, and increased TWIST expression shows enhanced migratory/invasive profiles (Fu et al., 2011; Hong et al., 2011) A previous study showed that knock down of TBX2 primarily inhibited the expression of the Twist gene (Du et al., 2017) . There is a high degree of homology between T-box transcription factor Tbx2 and the DNA-binding domain of Brachyury (Abrahams et al., 2010) , which is known as a prognostic marker of chordoma (Oakley et al., 2008) Wang et al. showed that TBX2 stimulates EMT and invasion of normal as well as malignant breast epithelial cells (Wang et al., 2012) . Silencing of TWIST might trigger metastasis in chordoma cells based on coexpression of TBX2 and TWIST. Therefore, we conclude that Twist might be a significant marker and a potential target to decrease the metastatic ability of chordoma. Suppression of TWIST might be used against metastatic chordoma.
References
- Abrahams A , Parker MI , Prince S ( 2010. ). The T-box transcription factor Tbx2: its role in development and possible implication in cancer . IUBMB Life 62 : 92 - 102 . [DOI] [PubMed] [Google Scholar]
- Bates RC , Mercurio AM ( 2005. ). The epithelial-mesenchymal transition (EMT) and colorectal cancer progression . Cancer Biol Ther 4 : 365 - 370 . [DOI] [PubMed] [Google Scholar]
- Bergh P , Kindblom LG , Gunterberg B , Remotti F , Ryd W , MeisKindblom JM ( 2000. ). Prognostic factors in chordoma of the sacrum and mobile spine - A study of 39 patients . Cancer 88 : 2122 - 2134 . [DOI] [PubMed] [Google Scholar]
- Chambers PW , Schwinn CP ( 1979. ). Chordoma. A clinicopathologic study of metastasis . Am J Clin Pathol 72 : 765 - 776 . [DOI] [PubMed] [Google Scholar]
- Chen ZF , Behringer RR ( 1995. ). Twist is required in head mesenchyme for cranial neural-tube morphogenesis . Gene Dev 9 : 686 - 699 . [DOI] [PubMed] [Google Scholar]
- Cheng GZ , Zhang W , Wang LH ( 2008. ). Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay . Cancer Res 68 : 957 - 960 . [DOI] [PubMed] [Google Scholar]
- Croset M , Goehrig D , Frackowiak A , Bonnelye E , Ansieau S , Puisieux A , Clezardin P ( 2014. ). TWIST1 expression in breast cancer cells facilitates bone metastasis formation . J Bone Miner Res 29 : 1886 - 1899 . [DOI] [PubMed] [Google Scholar]
- Du WL , Fang Q , Chen Y , Teng JW , Xiao YS , Xie P , Jin B , Wang JQ ( 2017. ). Effect of silencing the T-Box transcription factor TBX2 in prostate cancer PC3 and LNCaP cells . Mol Med Rep 16 : 6050 - 6058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles SA , Welch DR ( 2007. ). Metastasis: recent discoveries and novel treatment strategies . Lancet 369 : 1742 - 1757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J , Qin L , He T , Qin J , Hong J , Wong J , Liao L , Xu J ( 2011. ). The TWIST/Mi2/ NuRD protein complex and its essential role in cancer metastasis . Cell Res 21 : 275 - 289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J , Zhou J , Fu J , He T , Qin J , Wang L , Liao L , Xu J ( 2011. ). Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness . Cancer Res 71 : 3980 - 3990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber MA , Kraut N , Beug H ( 2005. ). Molecular requirements for epithelial-mesenchymal transition during tumor progression . Curr Opin Cell Biol 17 : 548 - 558 . [DOI] [PubMed] [Google Scholar]
- Karamchandani J , Wu MY , Das S , Vogel H , Muller P , Cusimano M , Montanera W , Kovacs K ( 2013. ). Highly proliferative sellar chordoma with unusually rapid recurrence . Neuropathology 33 : 424 - 430 . [DOI] [PubMed] [Google Scholar]
- Kwok WK , Ling MT , Lee TW , Lau TC , Zhou C , Zhang X , Chua CW , Chan KW , Chan FL , Glackin C et al. ( 2005. ). Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target . Cancer Res 65 : 5153 - 5162 . [DOI] [PubMed] [Google Scholar]
- Liu GL , Yang HJ , Liu T , Lin YZ ( 2014. ). Expression and significance of E-cadherin, N-cadherin, transforming growth factor-beta 1 and Twist in prostate cancer . Asian Pac J Trop Med 7 : 76 - 82 . [DOI] [PubMed] [Google Scholar]
- Markwalder TM , Markwalder RV , Robert JL , Krneta A ( 1979. ). Metastatic chordoma . Surg Neurol 12 : 473 - 478 . [PubMed] [Google Scholar]
- Matsuo N , Shiraha H , Fujikawa T , Takaoka N , Ueda N , Tanaka S , Nishina S , Nakanishi Y , Uemura M , Takaki A et al. ( 2009. ). Twist expression promotes migration and invasion in hepatocellular carcinoma . BMC Cancer 9 : 240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster ML , Goldstein AM , Bromley CM , Ishibe N , Parry DM ( 2001. ). Chordoma: incidence and survival patterns in the United States, 1973. - 1995 . Cancer Cause Control 12 : 1 - 11 . [DOI] [PubMed] [Google Scholar]
- McPherson CM , Suki D , McCutcheon IE , Gokaslan ZL , Rhines LD , Mendel E ( 2006. ). Metastatic disease from spinal chordoma: a 10-year experience . J Neurosurg-Spine 5 : 277 - 280 . [DOI] [PubMed] [Google Scholar]
- Oakley GJ , Fuhrer K , Seethala RR ( 2008. ). Brachyury, SOX-9, and podoplanin, new markers in the skull base chordoma vs chondrosarcoma differential: a tissue microarray-based comparative analysis . Modern Pathol 21 : 1461 - 1469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinner B , Froehlich EV , Buerger K , Knausz H , Lohberger B , Scheipl S , Fischer C , Leithner A , Guelly C , Trajanoski S et al. ( 2012. ). Establishment and detailed functional and molecular genetic characterisation of a novel sacral chordoma cell line, MUGChor1 . Int J Oncol 40 : 443 - 451 . [DOI] [PubMed] [Google Scholar]
- Thiery JP ( 2002. ). Epithelial-mesenchymal transitions in tumour progression . Nat Rev Cancer 2 : 442 - 454 . [DOI] [PubMed] [Google Scholar]
- Thiery JP ( 2003. ). Epithelial-mesenchymal transitions in development and pathologies . Curr Opin Cell Biol 15 : 740 - 746 . [DOI] [PubMed] [Google Scholar]
- Vanderheijden C ( 2014. ). Chordoma and its embryonic determinants . Marble Research Paper 308 : 150 - 159 . [Google Scholar]
- Vergara G , Belinchon B , Valcarcel F , Veiras M , Zapata I , de la Torre A ( 2008. ). Metastatic disease from chordoma . Clin Transl Oncol 10 : 517 - 521 . [DOI] [PubMed] [Google Scholar]
- Vesuna F , van Diest P , Chen JH , Raman V ( 2008. ). Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer . Biochem Bioph Res Co 367 : 235 - 241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B , Lindley LE , Fernandez-Vega V , Rieger ME , Sims AH , Briegel KJ ( 2012. ). The T box transcription factor TBX2 promotes epithelial-mesenchymal transition and invasion of normal and malignant breast epithelial cells . PLoS One 7 : e41355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J , Mani SA , Donaher JL , Ramaswamy S , Itzykson RA , Come C , Savagner P , Gitelman I , Richardson A , Weinberg RA ( 2004. ). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis . Cell 117 : 927 - 939 . [DOI] [PubMed] [Google Scholar]
- Yang J , Mani SA , Weinberg RA ( 2006. ). Exploring a new twist on tumor metastasis . Cancer Res 66 : 4549 - 4552 . [DOI] [PubMed] [Google Scholar]
- Yang MH , Chen CL , Chau GY , Chiou SH , Su CW , Chou TY , Peng WL , Wu JC ( 2009. ). Comprehensive analysis of the independent effect of Twist and Snail in promoting metastasis of hepatocellular carcinoma . Hepatology 50 : 1464 - 1474 . [DOI] [PubMed] [Google Scholar]
- Yang XR , Ng D , Alcorta DA , Liebsch NJ , Sheridan E , Li S , Goldstein AM , Parry DM , Kelley MJ ( 2009. ). T (Brachyury) gene duplication confers major susceptibility to familial chordoma . Nat Genet 41 : 1176 - 1178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z , Zhang X , Gang H , Li X , Li Z , Wang T , Han J , Luo T , Wen F , Wu X ( 2007. ). Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and bfironectin expression . Biochem Bioph Res Co 358 : 925 - 930 . [DOI] [PubMed] [Google Scholar]
- Zhou BP , Deng J , Xia W , Xu J , Li YM , Gunduz M , Hung MC ( 2004. ). Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition . Nat Cell Biol 6 : 931 - 940 . [DOI] [PubMed] [Google Scholar]