Abstract
Head and neck squamous cell carcinoma (HNSCC) is the most common and most aggressive type of head and neck cancer. Current approaches for the treatment of HNSCC are not sufficient to increase the patient survival or to reduce the high recurrence rate. Consequently, there is a need to explore the molecular characteristics of this cancer in order to discover potential therapeutic target molecules. The overexpression of chromosome region maintenance 1 (Crm1), responsible for the transport of different classes of macromolecules from the nuclear membrane to the cytoplasm, in various cancer cells has made it an attractive target molecule in cancer research. It has been reported that transcription factors, which are the target cargo proteins of Crm1, have critical roles in regulating intracellular processes via their expression levels and functions, which in turn are regulated by the cell cycle and signaling proteins. Previous findings show that head and neck cancer cells overexpress Crm1 and that these cells become highly dependent on Crm1 function. The results of this study show that after decreasing Crm1 expression levels in HNSCC cells through either treatment with specific Crm1 RNA interference (siRNA) or the selective Crm1 inhibitor leptomycin B (LMB), cell viability, proliferation, migration, and wound-healing abilities decreased, suppressing tumorigenic properties through the induction of apoptosis. Crm1 is a powerful diagnostic biomarker because of its central role in cancerogenesis, and it has a high potential for the development of targeted Crm1 molecules or synthetic agents, such as LMB, as well as for the improvement of the clinical features in head and neck cancer.
Keywords: Head and neck cancer, chromosome region maintenance 1, metastasis, RNA interference, leptomycin B
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer type and represents the third most common cause of cancer-related deaths worldwide (Stell et al., 1989; Jemal et al., 2009) . It constitutes 4% of all cancer cases, resulting in approximately 650,000 new cases and 400,000 deaths annually (Mao et al., 2004; Siegel et al., 2014) . In most cases of HNSCC, only 51% of short-term malignancies and only 10.5% of long-term malignancies could be detected even with advanced investigations. Five year survival rates are 51% in short-term malignancies and 28% in long-term malignancies (Jemal et al., 2009).
The underlying mechanism of HNSCC invasion and metastasis is a multistep process characterized by multiple genetic and molecular changes (Wilken et al., 2011) . However, not all of the underlying molecular mechanisms of HNSCC pathology are clear. Additionally, despite the standard therapies, including radiation, surgery, and/or chemotherapy, there has been no significant change in the survival rate within the last 20–30 years, and the mortality rate for HNSCC is still high (Jemal et al., 2009). Therefore, it is very important to investigate new candidate molecules for the diagnosis, follow-up, and control of HNSCC. Moreover, the investigation of potential target molecules that may be responsible for the HNSCC pathogenesis is crucial for the development of new clinical therapeutic approaches.
Chromosome region maintenance 1 (Crm1), a member of the cytoplasm-nucleus transport receptor family known as the karyopherins, is an important nuclear export protein in mammals that facilitates the transport of various classes of RNAs, proteins, and other macromolecules from the nuclear membrane to the cytoplasm, and it helps maintain their appropriate subcellular localization (Kudo et al., 1997; Nguyen et al., 2012; Turner et al., 2012) . Crm1 has a broad range of substrates and recognizes numerous cargo proteins, which are rich in nuclear export signal (NES) sequences, including tumor suppressor proteins such as p53, p27, and p21. These tumor suppressor proteins carry NES sequences rich in leucine amino acids and hydrophobic residues (Fukuda et al., 1997; Henderson et al., 2000; Mariano et al., 2006; Chan et al., 2010; van der Watt et al., 2011; Brodie et al., 2012; Santiago et al., 2013; Fung et al., 2014) . Furthermore, transcription factors that are the target cargo proteins of Crm1 have critical roles in the regulation of intracellular processes via their expression levels and functions, which are regulated by the cell cycle and signaling proteins (Henderson et al., 2000; Mariano et al., 2006; Chan et al., 2010; van der Watt et al., 2011; Brodie et al., 2012; Santiago et al., 2013) . The deregulation of Crm1 expression, which is dependent on the cell cycle, results in the loss of cellular proliferation control through various intracellular pathways (Ishizawa et al., 2015). Recent studies on various cancer types have reported an increase in the expression level of Crm1 compared with healthy tissue, and this increase has been found to be associated with metastasis, histological grading, increased tumor size, and a decreased general survival rate (Noske et al., 2008; Shen et al., 2009; van der Watt et al., 2009, 2014; Yao et al., 2009; Zhou et al., 2013; Tai et al., 2014; Yang et al., 2014; Liu et al., 2016) . The increased expression level of Crm1 has also been shown to play a key role in carcinogenesis, and it was observed that in retrovirus-mediated small interfering RNA (siRNA)-introduced Crm1 knockdown cancer lines, the proliferation and migration abilities of the cells were suppressed and apoptosis was induced (van der Watt et al., 2009, 2014; Yang et al., 2014) . Therefore, Crm1, a nuclear export molecule, has become a significantly promising target for the treatment of cancer (Yashiroda et al., 2003; Turner et al., 2011) . Leptomycin B (LMB) appeared as an efficient inhibitor molecule that blocks the function of the Crm1 protein. It has been reported that LMB irreversibly binds to the residue Cys528 in the ligand-binding domain of Crm1 and selectively inhibits this protein (Wolf et al., 1997; Kudo et al., 1999) . Preclinical studies using LMB as an anticancer agent are ongoing (Newlands et al., 1996) . Apoptotic pathways in cancer cells are activated by the specific Crm1-inhibitory function of LMB (Noske et al., 2008; van der Watt et al., 2009, 2014; Yang et al., 2014) .
The aim of this study was to investigate the potential role of Crm1 in head and neck cancer pathology, as well as to shed light on its potential as a therapeutic target. The effects of specific Crm1 knockdown and inhibition on cell proliferation, migration, and cellular apoptotic response in head neck cancer cells were investigated.
2. Materials and methods
2.1. Cell cultures
The following HNSCC cell lines were used for all experiments: UT-SCC-16A, UT-SCC-16B, UT-SCC-60A, UT-SCC-60B, UT-SCC-74, and UT-SCC-74B were kindly provided by Prof Dr Reidar Grenman (Department of Otorhinolaryngology-Head and Neck Surgery and Medical Biochemistry and Molecular Biology, Turku University and Turku University Central Hospital, Turku, Finland). All of them were originally established head and neck squamous cell carcinoma primary tumors (A series) and their associated metastatic tumors (B series). Characteristics of the cell lines are summarized in the Table. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/High Glucose (Cat# SH30243.01; HyClone, GE Healthcare, South Logan, UT, USA), supplemented with penicillin (100 U/mL), streptomycin (100 µg/mL) (Cat# SV30010; HyClone, GE Healthcare), 10% fetal bovine serum (FBS) (Cat# SV30160.03; HyClone, GE Healthcare), 0.8% L-glutamine (Cat# SH30034.01; HyClone, GE Healthcare), and 0.01% Plasmocin (ant-mpt; InvivoGen, San Diego, CA, USA). Cell lines were cultured at 37 °C in a humidified atmo sphere of 5% CO2.
Table.
Clinicopathological characteristics of the HNSCC cell lines.
| Accession ID | Cell line name | Sex of cell | Age | Primary tumor origin | TNM classification | Specimen site | Histological grade |
|---|---|---|---|---|---|---|---|
| CVCL_7812 | UT-SCC-16A | F | 77 | SCC, tongue | T3N0M0 | Tongue | G3 |
| CVCL_7813 | UT-SCC-16B | F | 77 | SCC, tongue | T3N0M0 | Neck | G3 |
| CVCL_A089 | UT-SCC-60A | M | 59 | Tonsil | T4N1M0 | Tonsil | G1 |
| CVCL_A090 | UT-SCC-60B | M | 59 | Tonsil | T4N1M0 | Neck | G1 |
| CVCL_7779 | UT-SCC-74A | M | 31 | SCC, tongue | T3N1M0 | Tongue | G1–G2 |
| CVCL_7780 | UT-SCC-74B | M | 31 | SCC, tongue | rN2 | Neck | G2 |
HNSCC: Head and neck cancer, M: male, F: female, TNM: TNM classification (T: tumor, N: lymph node involvement, M: distance metastases), SCC: squamous cell carcinoma.
2.2. LMB treatment
We used LMB (Cat# ab120501; Abcam, Cambridge, MA, USA) to test the effect of Crm1 inhibition on the apoptotic status, proliferation, and migration capability of head and neck cancer cells. LMB was stored as a 10.2 µM stock in ethanol. The cells were suspended in culture plates, preincubated at 37 °C overnight, and then treated for 48 h with different concentrations of LMB (0, 0.5, 1, 10, 20, and 40 nM).
2.3. RNA interference
All siRNAs were synthesized by GE Healthcare Dharmacon (Lafayette, CO, USA). For the inhibition of Crm1 gene expression siRNAs, ON-TARGETplus Human CRM1 siRNA-SMARTpool was used (Cat# L-003030-000005; GE Healthcare Dharmacon). siRNA consisting of a scrambled sequence from the ON-TARGETplus Human Non-targeting Control Pool (Cat# D-001810-10-05; GE Healthcare Dharmacon) was used as a nonsilencing control and GAPDH from the ON-TARGETplus Human GAPDH Control Pool (Cat# D-001830-10-05; GE Healthcare Dharmacon) was used as a control. Cells were seeded in complete media (without antibiotics) the day before the experiment (1–1.2 × 105 cells/well). Cell lines were transiently transfected with 25 nM siRNA into the cell lines using DharmaFECT-1 reagent (0.2 mL) (Cat# T-2001-01; GE Healthcare Dharmacon) according to the manufacturer’s protocol.
2.4. Quantitative real‑time reverse transcription‑PCR
RNA was isolated from the cell lines using TRIzol reagent (Cat# 15596026; Invitrogen, Rockville, MD, USA) and transcribed into cDNA using the Transcriptor High Fidelity cDNA Synthesis Kit (Cat# 05091284001; Roche Applied Science, Penzberg, Germany). The assays were performed in accordance with the manufacturer’s instructions. Quantitative real-time PCR was performed using the SYBR Green qPCR kit (Cat# 04887352001; Roche Applied Science) using the following primer pairs: Crm1 (F 5’ GGGAAAACTGAAACCCACCT 3’ and R 5’ CTGAAATCAAGCAGCTGACG 3’), betaactin (F 5’ TTCCTGGGCATGGAGTCCT 3’ and R 5’ AGGAGGAGCAATGATCTTGATC 3’), and GAPDH (F 5’ CAAGGTCATCCATGACAACTTTG 3’ and R 5’ GTCCACCACCCTGTTGCTGTAG 3’), where beta-actin and GAPDH were used to normalize for Crm1 expression. For qRT-PCR, the Rotor-Gene Q 5plex HRM Platform (QIAGEN, Hilden, Germany) was used and the data were analyzed using Rotor Gene Q Software 1.2 software (QIAGEN).
2.5. Western blot analysis
Cells in culture grown to 80% confluence were washed with precooled (4 °C) PBS (Cat# 51226; AccuGENE, Lonza, Walkersville, MD, USA) 3 times and lysed in radioimmunoprecipitation assay (RIPA) buffer (Cat# 89900; Thermo Scientific, Vernon Hills, IL, USA). Total proteins in the supernatant were collected. The protein concentrations were quantified by Bradford assay and 20 µg of total protein was used for western blot analysis. First 30 µL of each protein sample was mixed with 10 µL of 4X SDS sample buffer and separated by electrophoresis in an SDS-PAGE gel and transferred to polyvinylidene diufloride (PVDF) Hybond ECL nitrocellulose membranes (Cat# RPN2020D; GE Healthcare UK Limited, Amersham, UK).
For western blot analyses, the membranes were incubated at 4 °C overnight with primary antibodies against Crm1 (1/1000, Cat# ab24189; Abcam) and β-actin (1/20000, Cat#sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Then the membranes were subsequently incubated with horseradish peroxidase-linked secondary antibody anti-Crm1 rabbit IgG (1/3000, Cat# ab9705; Abcam) and anti-β-actin mouse IgG (1/2500, Cat #7076P2; Cell Signaling Technology, Danvers, MA, USA) at 37 °C for 1 h with shaking, and the bound proteins were visualized by ECL substrate (Cat# 1705060; Bio-Rad, Hercules, CA, USA) using the ChemiDoc MP Imaging System (BioRad). The relative intensities were evaluated with ImageJ software (https://imagej.net/Welcome).
2.6. Immunofluorescence analysis
The cells were first counted and 3 × 10 5 cells were seeded onto 13-mm coverslips (Nunc Thermanox, Cat# 174950; Thermo Scientific) for 24 h. At 48 h after transfection or inhibition, the medium was removed, and then cells were ifxed for 10 min with 4% formaldehyde (Cat# F8775; Sigma-Aldrich, St. Louis, MO, USA) in PBS at room temperature. Following 2 washes with PBS and fixing, cells were permeabilized in 0.5% Triton X-100 (Cat# 11332481001; Roche, Mannheim, Germany) in PBS for 10 min. After blocking with 1% BSA (Cat# 9048468; Sigma-Aldrich) in PBS for 30 min, cells were subsequently incubated with Crm1 primary antibodies (1/100 dilution, Cat# sc-5595-rabbit polyclonal antibody; Santa Cruz Biotechnology) in blocking buffer for 1 h. After 2 washes in PBS, cells were incubated with Alexa-Fluor 488-labeled secondary antibody for 30 min (1/200, Cat# Z25302; Life Technologies Corp., Carlsbad, CA, USA). After washing, cells were counterstained with 10 µg/mL diamido-2phenylindole dihydrochloride (DAPI) and coverslips were mounted with ProLong Gold Antifade Reagent (Cat# P36934; Life Technologies). Images were visualized using standard fluorescence microscopy.
2.7. Cell proliferation
The proliferation status of the cells was analyzed using the xCELLigence Real Time Cell Analyzer System (RTCA-DP) (Roche) and the (3-(4,5-dimethylthiazol-2yl)- 2,5-diphenyltetrazolium bromide) MTT assay. For the xCELLigence system proliferation assay, 100 mL of medium (DMEM) containing 2% FBS was added to the wells. After 1 h of equilibration with the medium, 100 mL of cell suspension (1–1.2 × 104 cells/well) was added to 96-well plates. Measurements were collected at an interval of 15 min and results were analyzed using the RTCA software. The monitored cell proliferation was expressed as percentage cell proliferation.
For the MTT cell proliferation assay, the cells were cultured separately onto 96-well plates (1–1.2 × 104 cells/ well), 24 h after transfection or inhibition. Brieyfl, the cells were incubated with Cell Proliferation Kit I (MTT) (Cat# 11465007001; Sigma-Aldrich) for 4 h at 37 °C in a humidified atmo sphere of 5% CO2, following the manufacturer’s instructions. After incubation for 48 h, the plates were read on a microplate reader (Variscan Flash Multimode Reader; Thermo Scientific) and the absorbance of the wells was measured at a wavelength of 595 nm.
2.8. Apoptosis assays
The apoptotic status of cells was investigated using caspase-activity with the Caspase 3 Activity Assay Kit (Cat# 12012952001; Roche Life Sciences, Indianapolis, IN, USA), according to the manufacturer’s instructions. Shortly, 1–1.2 × 104 cells were plated per well in 96-well plates and transfected with Crm1-siRNA or treated with LMB. Caspase activity was measured after 48 h, and luminescence was monitored using the Veritas Microplate Luminometer (Turner BioSystems, Sunnyvale, CA, USA).
2.9. Wound healing assay
Cells were cultured separately onto 12-well plates in fresh serum-free DMEM (1–1.2 × 105 cells/well) for 48 h. A wound was made in the middle of the wells using a sterile 200-µL micropipette tip and photographed using a Leica inverted microscope after 36 h (Cat# DM1000 DFC 295; Leica, Frankfurt, Germany), and the images were captured at 10× magnification.
2.10. Statistical analysis
Data from all the experiments are expressed as means from a minimum of 3 independent experiments. The Crm1 expression in HNSCC cell lines was analyzed by Mann–Whitney test. The rest of the data were statistically analyzed by Student’s t-test and P < 0.05 was required for statistical significance.
3. Results
3.1. Crm1 expression in primary and metastatic HNSCC cell lines
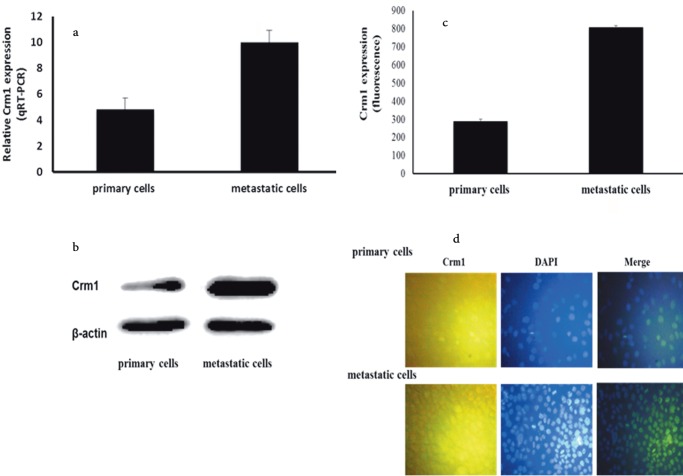
Crm1 expression levels in HNSCC cell lines were investigated in our study, since it was suggested that the increased expression of Crm1 was required for various cellular processes and was associated with the induction of specific tumorigenic properties (Noske et al., 2008; Yao et al., 2009; van der Watt et al., 2009, 2014; Shen et al., 2009; Zhou et al., 2013; Yang et al., 2014; Tai et al., 2014; Liu et al., 2016) . The qRT-PCR analysis showed a significant increase in Crm1 expression in all of the metastatic HNSCC cell lines compared to their primary cell lines, and the highest increase was observed in UT-SCC-74A and UT-SCC-74B cells (P < 0.05 for all; data not shown). It was also observed in the metastatic HNSCC cell lines that a significant increase in the relative Crm1 expression level was observed compared to the primary cell lines (approximately 2-fold; Figure 1a).
Figure 1.
Expression of Crm1 in HNSCC cell lines. (a) Relative Crm1 mRNA expression levels in HNSCC cell lines as determined by qRT-PCR. Relative mRNA expression levels are significantly upregulated in metastatic HNSCC cells [cancer cell lines (n = 6), P < 0.05] (scale bar, 200 μm). Results shown are the mean of 6 ± SE. (b) Western blot analysis confirming upregulation of Crm1 in metastatic HNSCC cells compared to primary cell lines (P < 0.05). Representative bands showing Crm1 protein expression in UT-SCC-74A and UT-SCC-74B cell lines. β-Actin was used as a control for protein loading. (c) Quantification of Crm1 immunofluorescence in 6 HNSCC cell lines. Fluorescence was quantified using ZEN software (Carl Zeiss Microscopy GmbH, Jena, Germany). A significant increase in Crm1 expression in metastatic HNSCC compared to primary cell lines [cancer cell lines (n = 6), P < 0.05]. (d) Immunohistochemical analysis of Crm1 expression in HNSCC cell lines. Elevated Crm1 expression in metastatic HNSCC compared to primary cancer cells was observed (P < 0.05). Merged images obtained with anti-Crm1 antibody and DAPI. Representative images showing Crm1 expression and nuclear staining in UT-SCC-74A and UT-SCC-74B cell lines. Crm1, Chromosome region maintenance 1 protein; DAPI, diamido- 2-phenylindole dihydrochloride.
The Crm1 protein expression level was investigated by western blot analysis after the detection of the increased level of mRNA expression of the CRM1 gene in the HNSCC cell lines. Similar to the qRT-PCR results, the UTSCC-74A and UT-SCC-74B cell lines demonstrated strong protein expression levels of Crm1 compared to other cell lines (Figure 1b). The metastatic UT-SCC-16B and UTSCC-60B cells showed higher expression levels of Crm1 compared to their primary counterparts, UT-SCC-16A and UT-SCC-60A.
To verify the Crm1 protein expression level increase, the cell lines were also analyzed by immunofluorescence. When primary and metastatic HNSCC cell lines were examined, the metastatic cells showed a high level of Crm1 protein expression as compared to the primary cells (Figure 1c).
In fluorescent images, Crm1 was mostly localized in the cytoplasm in primary and metastatic cells, although some nuclear expression was also detected and was compatible with the definition of the company producing the antibody (Figure 1d). Crm1 expression level results were found to be compatible with each other.
3.2. Crm1 inhibition by LMB decreases HNSCC cell viability and triggers apoptosis in vitro
In this study, increased levels of the CRM1 gene and protein expression levels were observed in metastatic HNSCC cells (Figure 1). We hypothesized that a decrease in the expression level of Crm1 in HNSCC cells, which plays a role in the critical intracellular processes underlying cancerogenesis, could prevent the occurrence of tumoral physiology. To understand the functional significance of increased Crm1 expression levels in metastatic HNSCC cells, we investigated its effect on cells in which its expression or function was inhibited.
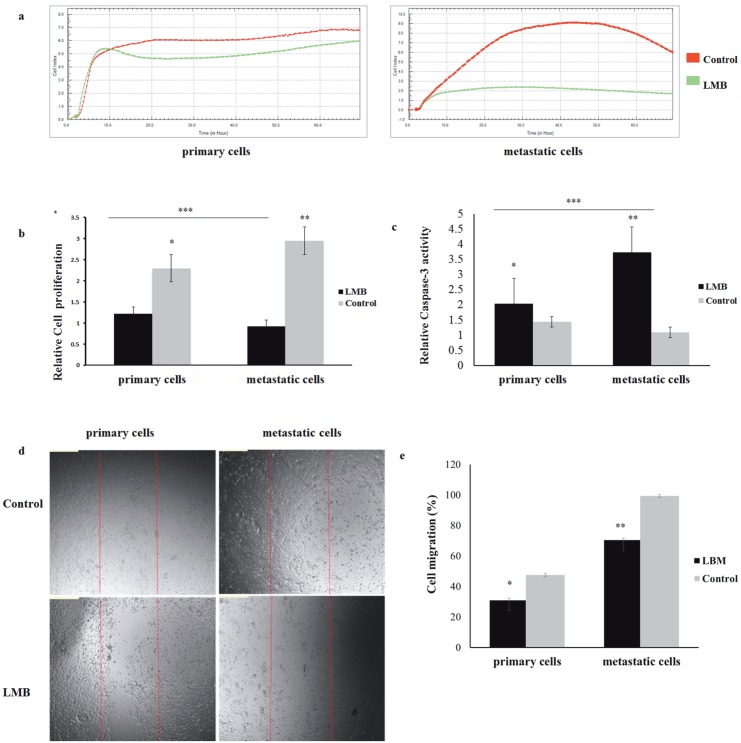
LMB is a specific Crm1 inhibitor that has previously been used in various studies in cancer cell lines and was used to inhibit Crm1 function in this study (Wolf et al., 1997; Kudo et al., 1999; Noske et al., 2008; Tai et al., 2014; van der Watt et al., 2014) . Analysis with the xCELLigence RTCA-DP system showed that, although there was a sensitivity to lower doses in the metastatic cell lines, the highest LMB sensitivity occurred between 10 and 20 nM (P < 0.05, for both primary and metastatic). In the primary cell lines, the highest sensitivity was observed at a concentration of 20 nM LMB (P < 0.05; data not shown). Moreover, metastatic cells are more sensitive to LMB treatment than primary HNSCC cells (Figure 2a). In this study, in contrast to the primary cells, the survival of metastatic head and neck cancer cells was shown to be closely related to Crm1 function.
Figure 2.
Effect of leptomycin B (LMB) treatment on cell viability, proliferation, and migration. (a) The primary UT-SCC-74A and metastatic UT-SCC-74B HNSCC cell lines were treated with LMB at increasing nanomolar concentrations and cell viability was assayed 48 h after treatment using the xCELLigence RTCA-DP system. Results shown are the cell index after LMB treatment relative to the untreated controls. HNSCC cell index in the absence and presence of 20 nM LMB. With LMB treatment metastatic HNSCC cells were decreased significantly in comparison to the control by using the xCELLigence RTCA-DP system (P < 0.05). (b) Relative cell proliferation was measured using MTT after 48 h of incubation in the absence or presence of 20 nM LMB. Cell proliferation in LMBtreated HNSCC cells showed a decreased (*P < 0.05, **P < 0.05, ***P < 0.05) (scale bar, 200 μm). (c) Caspase-3 activity in LMB-treated HNSCC cells. Cells were treated with 20 nM LMB and caspase-3 activity was analyzed as a measure of apoptosis. Caspase-3 activity in LMB-treated HNSCC cells was increased significantly in comparison to the control (*P < 0.05, **P < 0.05, ***P < 0.05) (scale bar, 200 μm). (d) Wound-healing assay in the absence or presence of 5 nM LMB. Wound-healing assay showing that LMB was effective at disrupting the migration ability of HNSCC cells (for both P < 0.05) (scale bar, 50 μm). (e) Cell migration in LMB-treated HNSCC cells showed a decrease (*P < 0.05, **P < 0.05) (scale bar, 200 μm).
Furthermore, the proliferation abilities of LMB-treated HNSCC cancer cells were analyzed by MTT assay. A significant increase in the cell death rate was observed in HNSCC cancer cells treated with LMB. It was observed that the proliferation ability of metastatic HNSCC cells treated with LMB was suppressed more than that of the primary cells (Figure 2b). Additionally, in the caspase-3 assay, caspase-3 activity was observed in HNSCC cells treated with LMB, and it was also observed that activation in the metastatic cells was increased about 3-fold more than in the primary cells. These data revealed that the functional inhibition of the Crm1 protein activated apoptotic pathways, leading to tumor cell death (Figure 2c). Due to the remarkable effects of LMB at doses of 1020 nM on cell death in cancer cells, LMB at 5 nM was used to show the suppression of migration (Figure 2d). The migration of HNSCC cells treated with LMB was found to be decreased by approximately 1.5-fold in primary cell lines and 1.3-fold in metastatic cell lines (Figure 2e).
3.3. Crm1 knockdown by specific siRNA decreases HNSCC cell proliferation and induces apoptosis
By using specific siRNA for inhibiting the Crm1 expression in HNSCC cells, the effect of knockdown on cellular functions was investigated. HNSCC cancer cells were transfected with Crm1-specific siRNA or nontargeting (NT) control siRNA. The knockdown efficiency was assessed by qRT-PCR analysis and western blot.
The qRT-PCR analysis showed that Crm1 mRNA levels were significantly decreased in Crm1-siRNA-transfected cells compared to the NT control (Figure 3a). Proteins were harvested 72 h after transfection, and the cell lysates were analyzed by western blot using anti-Crm1 and antiβ-actin antibodies. Western blot results demonstrated that the Crm1 protein was significantly suppressed compared to the nontargeting control in both cell lines (Figure 3b).
Figure 3.
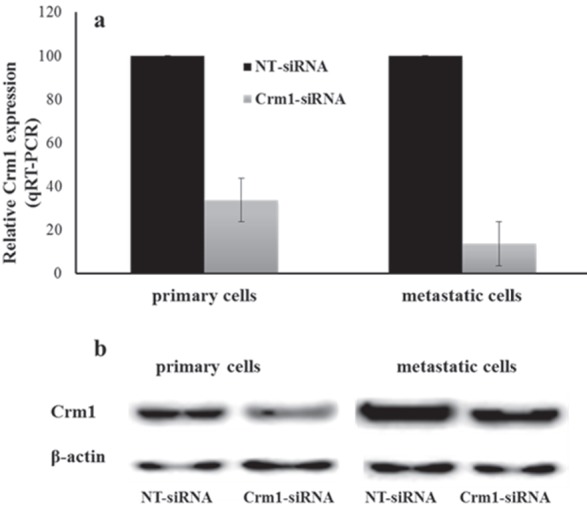
Knockdown efficiency of Crm1 in HNSCC cell lines. Crm1-siRNA and NT-siRNA were transfected in UT-SCC-74A and UT-SCC-74B cells. (a) After 48 h of transfection, the results showed that Crm1-siRNA compared to the NT-siRNA control led to a 66.3% decrease for primary HNSCC cells and 86.5% decrease for metastatic cells in the level of Crm1 mRNA by RT-qPCR (for both P < 0.001) (scale bar, 200 μm). (b) Western blot analysis demonstrated that Crm1 was suppressed significantly at the protein level in Crm1-siRNA compared to the NT-siRNA control in HNSCC cells (for both P < 0.001).
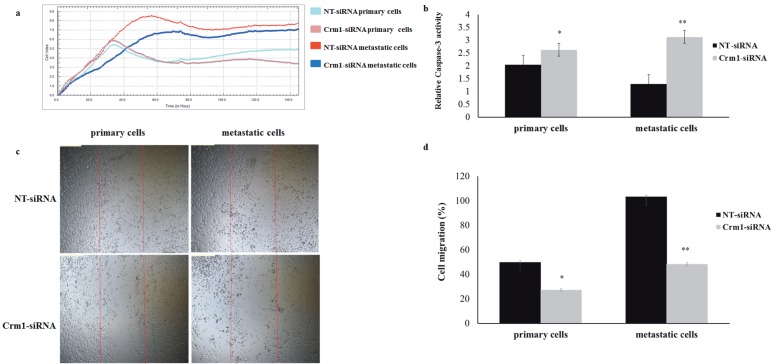
To test the inhibitory effect of Crm1 suppression on HNSCC cell proliferation, a proliferation assay using the xCELLigence system was performed 48 h after transfection. Cell index values for each treatment group at 20, 40, 60, and 120 h were compared. After Crm1 knockdown, the number of Crm1-siRNA-treated HNSCC cancer cells was significantly decreased compared to cells treated with the NT-siRNA (Figure 4a).
Figure 4.
Effect of inhibiting Crm1 expression on HNSCC cell viability, proliferation, and migration using siRNA. (a) Cell proliferation assays were performed using the xCELLigence RTCA-DP system. The primary UT-SCC-74A and metastatic UT-SCC-74B HNSCC cell lines were transfected with siRNA and cell viability was monitored 120 h after transfection. The primary and metastatic HNSCC cells showed no similar sensitivities to Crm1 inhibition. In primary cells, proliferation decreased by Crm1-siRNA after 80 h (P < 0.05). The metastatic HNSCC cancer cells were significantly reduced by Crm1-siRNA after 20 h (P < 0.05). (b) Caspase-3 activity in Crm1-knockdown HNSCC cells. Cells were in Crm1-siRNA transfection-activated apoptosis in the HNSCC cells compared to the nontargeting siRNA‑treated (*P < 0.05, **P < 0.001) (scale bar, 300 μm). (c) In HNSCC cells, there was significant difference between the Crm1-siRNA- and NT-siRNA-treated cells in terms of migration capability (for both P < 0.05) (scale bar, 50 μm). (e) Cell migration in Crm1-siRNA-treated HNSCC cells showed a decrease (*P < 0.05, **P < 0.05) (scale bar, 200 μm).
In Crm1 knockdown HNSCC cells, the caspase-3 activity was evaluated in order to see whether Crm1 suppression induced apoptosis and reduced the cell proliferation ability. Compared to the NT control, the caspase-3 activity in HNSCC cells transfected with Crm1specific siRNA was found to be increased by approximately 0.6-fold in primary cell lines and 2.3-fold in metastatic cell lines. These results may indicate that inhibiting the CRM1 gene expression triggers cell death in cancer cells (Figure 4b). In addition, the effect of Crm1 knockdown by siRNA on the migration and wound-healing capacity of HNSCC cells was investigated and it was observed that Crm1 cell migration was significantly decreased (Figure 4c). The migration of HNSCC cells transfected with Crm1-specific siRNA was found to be decreased by 1.8-fold in primary cell lines and 2.12-fold in metastatic cell lines (Figure 4d).
4. Discussion
Head and neck cancer is aggressive and highly metastatic, and the primary treatment option is surgery. Surgical treatments, due to their outcomes, significantly decrease the quality of life of the patients (Jemal et al., 2009). Similarly, due to the high toxicity and the intracellular drug resistance mechanisms that develop over time for other available therapeutic options, the 5-year survival rate of head and neck cancer is low (Mao et al., 2004; Siegel et al., 2014) . For this reason, there is an urgent need to investigate the molecular characteristics of head and neck cancer and to discover new therapeutic target molecules associated with specific cellular pathways to overcome these potential drug resistance mechanisms.
Crm1 is very critical for the cell cycle and proliferation, and it is responsible for the nuclear export of more than 285 critical proteins involved in intracellular processes (Fukuda et al., 1997; Nguyen et al., 2012; Turner et al., 2012; Fung et al., 2014) . Furthermore, transcription factors that are the target cargo proteins of Crm1 have critical roles in the regulation of intracellular processes via their expression levels and functions, which are regulated by the cell cycle and signaling proteins (Henderson et al., 2000; Mariano et al., 2006; Chan et al., 2010; van der Watt et al., 2011; Brodie et al., 2012; Santiago et al., 2013; Ishizawa et al., 2015) . It has been reported that Crm1 is expressed in different cell types, its level of expression changes in various pathologies, and its deregulation may lead to increased cell proliferation and decreased cell death (Kudo et al., 1997; Ishizawa et al., 2015).
For the first time, a study was conducted that showed an increased expression of Crm1 in cancerous tissues, and the results of this study indicated a possible relationship between Crm1 and cancer (van der Watt et al., 2009) . Additionally, it was shown in advanced studies that the increased expression of Crm1 was verified in 17 different tumor types, including multiple myeloma (Tai et al., 2014) , ovarian cancer (Noske et al., 2008) , osteosarcoma (Yao et al., 2009) , esophageal cancer (van der Watt et al., 2014; Yang et al., 2014) , glioma (Shen et al., 2009) , gastric cancer (Zhou et al., 2013) , and renal cancer (Liu et al., 2016) . This overexpression was correlated with poor patient survival and was reported to have a negative prognostic value (Turner et al., 2011) . Therefore, recent studies, especially on various types of cancer, have brought Crm1 to the foreground as an important target molecule (Yashiroda et al., 2003; Turner et al., 2011) .
It was also reported that cell cycle inhibitors involved in the pathogenesis of cancer were targeted by Crm1; tumor suppressor proteins, including BRAC1, the FOXOs, Rb, APC, RASSF2, Merlin, nucleophosmin, survivin, STAT, a κB-α inhibitor (IκB-α), and transcription factors NFY/ CBP, Sp1, and p53, are all aefcted by Crm1 overexpression and contribute to cellular transformations together with Crm1. Crm1 deregulation has been demonstrated mainly in cervical squamous cell carcinoma (van der Watt et al., 2009) , though also in many other cancer types, such as esophageal cancer (van der Watt et al., 2014; Yang et al., 2014) , multiple myeloma (Tai et al., 2014) , and renal cancer (Liu et al., 2016) , and it has been shown that Crm1 inhibition has the potential to target continuously proliferating cancer cells (Kau et al., 2004). Crm1-targeting agents containing inhibitors, such as LMB (Newlands et al., 1996; Wolf et al., 1997; Kudo et al., 1999) , N-azolylacrylate analogs (Daelemans et al., 2002) , Valeriana fauriei and Alpinia galanga l (Tamura et al., 2009) , the FOXO family (Kau et al., 2003), and the novel SINE (Sakakibara et al., 2011) , are highly potent candidate molecules that could be used to develop anticancer therapies when the role of Crm1 is confirmed. Today, more data are needed to reveal the role of Crm1 and its association to cellular pathways in order to clarify the pathogenesis of HNSCC and to develop effective treatment strategies.
In the HNSCC cell lines, we detected the expression of nuclear export protein Crm1, which is a member of the karyopherin family. We found that Crm1 is increasingly expressed in metastatic cells compared to primary HNSCC cells. To our knowledge, this is the first in vitro study reporting the expression of Crm1 in HNSCC cell lines. It also supports the idea that Crm1 might play a role in cancerogenesis since its suppression reduced cell growth and migration, potentially making it a potent biomarker in HNSCC diagnostics and treatment (Noske et al., 2008; Shen et al., 2009; van der Watt et al., 2009, 2014; Yao et al., 2009; Zhou et al., 2013; Tai et al., 2014; Yang et al., 2014; Liu et al., 2016) . Another important first in our study is the examination of mRNA and protein expression levels in primary HNSCC cells and their metastatic cell lines. UTSCC-74A, a primary tumor cell line, and UT-SCC-74B, its corresponding metastatic tumor cell line, demonstrated increased mRNA and protein expression levels of Crm1 compared to other cell lines. This difference may be due to the different phenotypic characteristics in UT-SCC-74A and -74B cells, such as clinicopathologic classification, histological grading, and an already high migration and metastatic capacity related to other acquired genetic changes (Sheikh Ali et al., 2008; Lange et al., 2009; Maushagen et al., 2016; Bender et al., 2018) .
We have taken 2 approaches to understanding the functional association between HNSCC cells and Crm1, which plays a role in the critical intracellular processes underlying cancerogenesis, and have determined the potential of Crm1 to be a therapeutic target. In order to suppress Crm1 gene expression, we used specific siRNA interference, and to inhibit protein function, we used a Crm1-targeting drug, LMB. Similar to LMB inhibition, when the siRNA approach was used, Crm1 expression levels decreased in the Crm1 knockdown cells and some of the tumorigenic properties of the HNSCC cells were altered via the induction of apoptosis. Additionally, decreases in cellular proliferation, migration, and the wound-healing abilities of HNSCC cells were observed.
LMB covalently binds to Crm1-specific cysteine residues and inhibits Crm1 binding to target cargo molecules (Wolf et al., 1997; Kudo et al., 1999) . In the study of van der Watt et al. (2009) performed on cervical cancer cells, it was reported that transformed cells were more sensitive to LMB treatment than normal cells, and LMB was highly cytotoxic at nanomolar concentrations. It is possible that the difference in sensitivity to LMB was related to the variability in the proliferation and metabolism of cancerous and normal cells. Kuusisto et al. (2011) suggested that to sustain the high proliferative abilities of transformed cancer cells and to maintain their metabolic activities, these cells required the increased expression and higher activity of proteins, such as Crm1, and therefore might be more sensitive to LMB than normal cells. We presented herein that metastatic cancer cells were much more susceptible to lower doses of LMB therapy compared to primary HNSCC cells, and we believe that the difference in response to Crm1 inhibition is promising in the development of Crm1-targeted anticancer drugs.
In our study, a significant reduction in the cell growth rate and migration was observed after Crm1 inhibition and knockdown in HNSCC cells. Previous studies have found that MMP2 and MMP9 expression levels are associated with an increased proliferation and metastatic ability of HNSCC cells (Kaomongkolgit et al., 2013; Chan et al., 2016) . Recently, it was shown that Crm1 inhibition in epithelial ovarian carcinoma cells reduced MMP2 and MMP9 levels and their enzymatic activities, possibly through mTOR-STAT3 signaling (Shao et al., 2017) . Therefore, in our study, we suggested that Crm1 inhibition may result in a decrease in the activity of MMP2 and MMP9 in cells, leading to a decrease in the migration and wound-healing capacity of HNSCC cells; however, further functional investigations are needed.
In addition, serine protease 6 (Ser6), associated with cell death, induces phosphorylated galectin-3 (Gal-3) to initiate cell cycle arrest and protects the cells from apoptosis (Yoshii et al., 2002) . Crm1 is responsible for the nuclear export of phosphorylated Ser6-phosphorylatedGal-3, and the overactivity of Crm1 in breast carcinoma cells causes excessive cytoplasmic accumulation of Gal3, resulting in antiapoptotic activity leading to cancer progression (Takenaka et al., 2004) . However, in one of the previous studies on HNSCC cells, it was reported that high levels of Gal-3 in tumor cells led to a cytoplasmic shift, and these cells developed apoptosis resistance (Saussez et al., 2008) . The observed increase in apoptotic response following Crm1 inhibition and Crm1 knockdown in this study could have resulted in the loss of other abnormal oncogenic regulatory mechanisms induced by the increased expression of Crm1, as well as causing a change in this pathway.
Head and neck carcinomas compose more than 90% of all squamous cell carcinomas. HNSCC has a high prevalence of neck metastases before spreading to distant regions (Easty et al., 1981) . Currently, up to 300 HNSCC cell lines have been established, and according to the location of the tumor, these are classified into 7 groups. Most, however, originate from the oral cavity (Lin et al., 2007) . Our study does have some limitations. Although the cell lines used herein do represent the general characteristics of head and neck tumors, they may have different genetic and phenotypic characteristics from other lines. Therefore, our data need to be confirmed in other head and neck cancer cell lines. In addition, our in vitro study has yet to demonstrate that Crm1 plays an important role in cell proliferation and migration in carcinogenesis, and despite the theoretical data, in vivo studies revealing the expression and molecular associations of Crm1 are still lacking. Our study shows only the effects of Crm1 by siRNA knockdown on primary and metastatic head and neck cancer cell lines. For this reason, Crm1 expression needs to be investigated in one or more normal cell lines and to be compared in normal and head neck cancer cells, and also in a clinical setting, including through the measurement of expression levels in patient plasma as well as in situ and in migrated tumor specimens to verify its impact on the prognosis.
In conclusion, this study has demonstrated the phenotypic effects of Crm1 on head and neck cancer cells, revealing an ability of this protein to induce proliferation and migration in these head and neck cancer cells. The current study opens up the potential use of Crm1 as a biological marker and a therapeutic target in the diagnosis and treatment of head and neck cancer. Further studies are needed to clarify and confirm this potential.
Acknowledgments
This study was financially supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project No. 114S948) and presented at the 2nd International Mediterranean Science and Engineering Congress, Adana, Turkey, 2017.
References
- Bender O , Gunduz M , Cigdem S , Hatipoglu OF , Acar M , Kaya M , Grenman R , Gunduz E , Ugur KS ( 2018. ). Functional analysis of ESM1 by siRNA knockdown in primary and metastatic head and neck cancer cells . J Oral Pathol Med 47 : 40 - 47 . [DOI] [PubMed] [Google Scholar]
- Brodie KM , Henderson BR ( 2012. ). Characterization of BRCA1 protein targeting, dynamics, and function at the centrosome. A role for the nuclear export signal, CRM1, and Aurora A kinase . J Biol Chem 287 : 7701 - 7716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY , Lien CH , Lee MF , Huang CY ( 2016. ). Quercetin suppresses cellular migration and invasion in human head and neck squamous cell carcinoma (HNSCC) . Biomedicine (Taipei) 6 : 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KS , Wong CH , Huang YF , Li HY ( 2010. ). Survivin withdrawal by nuclear export failure as a physiological switch to commit cells to apoptosis . Cell Death Dis 1 : e57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daelemans D , Afonina E , Nilsson J , Werner G , Kjems J , De Clercq E , Pavlakis GN , Vandamme AM ( 2002. ). A synthetic HIV-1 Rev inhibitor interfering with the CRM1-mediated nuclear export . P Natl Acad Sci USA 99 : 14440 - 14445 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easty D , Easty G , Carter R , Monaghan P , Butler L ( 1981. ). Ten human carcinoma cell lines derived from squamous carcinomas of the head and neck . Br J Cancer 43 : 772 - 785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M , Asano S , Nakamura T , Adachi M , Yoshida M , Yanagida M , Nishida E ( 1997. ). CRM1 is responsible for intracellular transport mediated by the nuclear export signal . Nature 390 : 308 - 311 . [DOI] [PubMed] [Google Scholar]
- Fung HY Chook YM Atomic basis of CRM1-cargo recognition, release and inhibition. Semin Cancer Biol. 2014;27:5261. doi: 10.1016/j.semcancer.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BR Nuclear cytoplasmic shuttling of APC regulates beta catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- Ishizawa J Kojima K Hail N Jr Tabe Y Andreef M Expression, function, and targeting of the nuclear exporter chromosome region maintenance 1 (CRM1) protein. Pharmacol Ther. 2015;153:25–35. doi: 10.1016/j.pharmthera.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A Siegel R Ward E Hao Y Xu J Thun MJ Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Kaomongkolgit R Alpha-mangostin suppresses MMP-2 and MMP-9 expression in head and neck squamous carcinoma cells. Odontology. 2013;101:227–232. doi: 10.1007/s10266-012-0081-2. [DOI] [PubMed] [Google Scholar]
- Kau TR Schroeder F Ramaswamy S Wojciechowski CL Zhao JJ Roberts TM Clardy J Sellers WR Silver PA A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-defficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Kau TR Way JC Silver PA Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- Kudo N Khochbin S Nishi K Kitano K Yanagida M Yoshida M Horinouchi S Molecular cloning and cell cycle dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- Kudo N , Matsumori N , Taoka H , Fujiwara D , Schreiner, EP , Wolf B , Yoshida M , Horinouchi S ( 1999. ). Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region . P Natl Acad Sci USA 96 : 9112 - 9117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusisto HV , Wagstaf KM , Alvisi G , Roth DM , Jans DA ( 2011. ). Global enhancement of nuclear localization-dependent nuclear transport in transformed cells . FASEB J 26 : 1181 - 1193 . [DOI] [PubMed] [Google Scholar]
- Lange MJ , Lasiter JC , Misfeldt ML ( 2009. ). Toll-like receptors in tonsillar epithelial cells . Int J Pediatr Otorhinolaryngol 73 : 613 - 621 . [DOI] [PubMed] [Google Scholar]
- Lin CJ , Grandis JR , Carey TE , Gollin SM , Whiteside TL , Koch WM , Ferris RL , Lai SY ( 2007. ). Head and neck squamous cell carcinoma cell lines: established models and rationale for selection . Head Neck 29 : 163 - 188 . [DOI] [PubMed] [Google Scholar]
- Liu X , Chong Y , Liu H , Han Y , Niu M ( 2016. ). CRM1 inhibitor S109 suppresses cell proliferation and induces cell cycle arrest in renal cancer cells . Korean J Physiol Pharmacol 20 : 161 - 168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L , Hong WK , Papadimitrakopoulou VA ( 2004. ). Focus on head and neck cancer . Cancer Cell 5 : 311 - 316 . [DOI] [PubMed] [Google Scholar]
- Mariano AR , Colombo E , Luzi L , Martinelli P , Volorio S , Bernard L , Meani N , Bergomas R , Alcalay M , Pelicci PG ( 2006. ). Cytoplasmic localization of NPM in myeloid leukemias is dictated by gain of function mutations that create a functional nuclear export signal . Oncogene 25 : 4376 - 4380 . [DOI] [PubMed] [Google Scholar]
- Maushagen R , Reers S , Pfannerstill AC , Hahlbrock A , Stauber R , Rahmanzadeh R , Rades D , Pries R , Wollenberg B ( 2016. ). Eefcts of paclitaxel on permanent head and neck squamous cell carcinoma cell lines and identification of anti-apoptotic caspase 9b . J Cancer Res Clin Oncol 142 : 1261 - 1271 . [DOI] [PubMed] [Google Scholar]
- Newlands ES , Rustin GJ , Brampton MH ( 1996. ). Phase I trial of elactocin . Br J Cancer 74 : 648 - 649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT , Holloway MP , Altura RA ( 2012. ). The XPO1 nuclear export protein in normal development and disease . Int J Biochem Mol Biol 3 : 137 - 151 . [PMC free article] [PubMed] [Google Scholar]
- Noske A , Weichert W , Niesporek S , Roske A , Buckendahl AC , Koch I , Sehouli J , Dietel M , Denkert C ( 2008. ). Expression of the nuclear export protein chromosomal region maintenance/ exportin 1/Xpo1 is a prognostic factor in human ovarian cancer . Cancer 112 : 1733 - 1743 . [DOI] [PubMed] [Google Scholar]
- Sakakibara K , Saito N , Sato T , Suzuki A , Hasegawa Y , Friedman JM , Kufe DW , Vonhof DD , Iwami T , Kawabe T ( 2011. ). CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity . Blood 118 : 3922 - 3931 . [DOI] [PubMed] [Google Scholar]
- Santiago A , Li D , Zhao LY , Godsey A , Liao D ( 2013. ). p53 SUMOylation promotes its nuclear export by facilitating its release from the nuclear export receptor CRM1 . Mol Biol Cell 24 : 2739 - 2752 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saussez S , Decaestecker C , Mahillon V , Cludts S , Capouillez A , Chevalier D , Vet HK , André S , Toubeau G , Leroy X et al. ( 2008. ). Galectin-3 upregulation during tumor progression in head and neck cancer . Laryngoscope 118 : 1583 - 1590 . [DOI] [PubMed] [Google Scholar]
- Shao WY , Yang YL , Yan H , Huang Q , Liu KJ , Zhang S ( 2017. ). Phenethyl isothiocyanate suppresses the metastasis of ovarian cancer associated with the inhibition of CRM1-mediated nuclear export and mTOR-STAT3 pathway . Cancer Biol Ther 18 : 26 - 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh Ali MA , Gunduz M , Nagatsuka H , Gunduz E , Cengiz B , Fukushima K , Beder LB , Demircan K , Fujii M , Yamanaka N et al. ( 2008. ). Expression and mutation analysis of epidermal growth factor receptor in head and neck squamous cell carcinoma . Cancer Sci 99 : 1589 - 1594 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A , Wang Y , Zhao Y , Zou L , Sun L , Cheng C ( 2009. ). Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis . Neurosurgery 65 : 153 - 159 . [DOI] [PubMed] [Google Scholar]
- Siegel R , Ma J , Zou Z , Jemal A ( 2014. ). Cancer statistics, 2014. . CA Cancer J Clin 64 : 9 - 29 . [DOI] [PubMed] [Google Scholar]
- Stell PM ( 1989. ). Survival times in end-stage head and neck cancer . Eur J Surg Oncol 15 : 407 - 410 . [PubMed] [Google Scholar]
- Tai YT , Landesman Y , Acharya C , Calle Y , Zhong MY , Cea M , Tannenbaum D , Cagnetta A , Reagan M , Munshi AA et al. ( 2014. ). CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications . Leukemia 28 : 155 - 165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka Y , Fukumori T , Yoshii T , Oka N , Inohara H , Kim HR , Bresalier RS , Raz A ( 2004. ). Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs . Mol Cell Biol 24 : 4395 - 4406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S , Shiomi A , Kaneko M , Ye Y , Yoshida M , Yoshikawa M , Kimura T , Kobayashi M , Murakami N ( 2009. ). New Revexport inhibitor from Alpinia galanga and structure-activity relationship . Bioorg Med Chem Lett 19 : 2555 - 2557 . [DOI] [PubMed] [Google Scholar]
- Turner JG , Dawson J , Sullivan DM ( 2011. ). CRM1-mediated nuclear export of proteins and drug resistance in cancer . Curr Med Chem 15 : 2648 - 2655 . [DOI] [PubMed] [Google Scholar]
- Turner JG , Dawson J , Sullivan DM ( 2012. ). Nuclear export of proteins and drug resistance in cancer . Biochem Pharmacol 83 : 1021 - 1032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Watt PJ , Leaner VD ( 2011. ). The nuclear exporter, Crm1, is regulated by NFY and Sp1 in cancer cells and repressed by p53 in response to DNA damage . Biochim Biophys Acta 1809. : 316 - 326 . [DOI] [PubMed]
- van der Watt PJ , Maske CP , Hendricks DT , Parker MI , Denny L , Govender D , Birrer MJ , Leaner VD ( 2009. ). The Karyopherin proteins, Crm1 and Karyopherin β1, are overexpressed in cervical cancer and are critical for cancer cell survival and proliferation . Int J Cancer 124 : 1829 - 1840 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Watt PJ , Zemanay W , Govender D , Hendricks DT , Parker MI , Leaner VD ( 2014. ). Elevated expression of the nuclear export protein, Crm1 (exportin 1), associates with human oesophageal squamous cell carcinoma . Oncol Rep 32 : 730 - 738 . [DOI] [PubMed] [Google Scholar]
- Wilken B , Veena MS , Wang MB , Srivatsan ES ( 2011. ). Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma . Mol Cancer 10 : 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B , Sanglier JJ , Wang Y ( 1997. ). Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodefficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA . Chem Biol 4 : 139 - 147 . [DOI] [PubMed] [Google Scholar]
- Yang X , Cheng L , Yao L , Ren H , Zhang S , Min X , Chen X , Zhang J , Li M ( 2014. ). Involvement of chromosome region maintenance 1 (CRM1) in the formation and progression of esophageal squamous cell carcinoma . Med Oncol 31 : 155 . [DOI] [PubMed] [Google Scholar]
- Yao Y , Dong Y , Lin F , Zhao H , Shen Z , Chen P , Sun YJ , Tang LN , Zheng SE ( 2009. ). The expression of CRM1 is associated with prognosis in human osteosarcoma . Oncol Rep 21 : 229 - 235 . [PubMed] [Google Scholar]
- Yashiroda Y , Yoshida M ( 2003. ). Nucleo-cytoplasmic transport of proteins as a target for therapeutic drugs . Curr Med Chem 10 : 741 - 748 . [DOI] [PubMed] [Google Scholar]
- Yoshii T , Fukumori T , Honjo Y , Inohara H , Kim HR , Raz A ( 2002. ). Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest . J Biol Chem 277 : 6852 - 6857 . [DOI] [PubMed] [Google Scholar]
- Zhou F , Qiu W , Yao R , Xiang J , Sun X , Liu S , Lv J , Yue L ( 2013. ). CRM1 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas . Med Oncol 30 : 726 . [DOI] [PubMed] [Google Scholar]