Japanese encephalitis (JE) is one of the most important etiologies of viral encephalitis. The classical manifestation of JE is parkinsonism, such as mask‐like face, rigidity, and tremor.1 However, acute flaccid paralysis can be a presenting symptom.2 JE is frequently accompanied by a variety of movement disorders, including facial grimacing, lip smacking, and dystonia.1, 3 Here, we describe a case of JE presenting with unusually slow mandibulo‐faciolingual tremor.
Case Report
A 79‐year‐old woman without any significant past medical history developed a fever up to 40°C and vomiting on a late summer day. On the next day, she had weakness of the lower limbs, and her consciousness began to deteriorate on the following day. At admission, she displayed a Glasgow Coma Scale score of 13 (E4V4M5). Nuchal rigidity was absent. Her muscle tone was universally decreased, and her tendon reflexes were lost in all four limbs. Babinski's sign was positive on the left side. She did not present with any apparent cerebellar ataxia or ophthalmoplegia. Cerebrospinal fluid (CSF) analysis revealed 273 cells/mm3 (75% polymorphonucleocytes and 25% lymphocytes) and elevated protein levels. A peripheral blood test demonstrated moderate leukocytosis, but was otherwise normal. On the following day, she exhibited shallow breathing with carbon dioxide retention; the day after that, she developed respiratory arrest and was intubated. At that point, she was in a comatose state, and light and oculocephalic reflexes were absent. She also exhibited flaccid tetraparalysis. A follow‐up CSF analysis revealed 102 cells/mm3 (all lymphocytes). Nerve conduction studies indicated that she experienced motor‐dominant axonal neuropathy. An MRI study revealed bilateral T2 hyperintensity in the substantia nigra (SN) (Fig. 1). She received pulse corticosteroid therapy and intravenous immunoglobulin (IVIg) because of the possibility of immune‐mediated encephalitis; however, the patient did not respond to these therapies. Fourteen days after the onset of symptoms, she developed a slow (approximately 0.6 Hz), rhythmic, continuous, uniform abnormal movement that affected her tongue, mandible, and right‐lower facial muscles (see Video). The movement became apparent when her eyes were open or after painful stimuli and was diminished when her eyes were closed. No abnormal movements were observed in the palate or limbs. Additionally, fiberscopic examination revealed no involuntary movements in the larynx. EEG revealed no epileptic discharges. Subsequently, she was diagnosed with JE based on a positive result for the JE virus by real‐time reverse‐transcriptase polymerase chain reaction and JE virus‐specific immunoglobulin M (IgM) antibodies (Abs) by IgM‐captured enzyme‐linked immunosorbent assay in both serum and CSF samples. The amplitude of abnormal movements became gradually decreased and disappeared spontaneously at approximately 80 days after onset.
Figure 1.
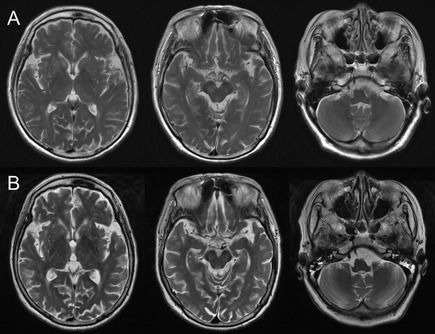
T2‐weighted MRI of the brain. (A) MRI at the time of admission. Bilateral hyperintense areas with unclear margins are detected in the substantia nigra (SN). (B) The bilateral T2 hyperintensity in the SN becomes defined at 46 days after onset. The thalamus and basal ganglia are minimally involved, and the inferior olive and cerebellum are preserved.
Discussion
The patient presented with very slow mandibulo‐faciolingual wiggling tremor in the course of JE, which disappeared without specific treatments. The phenomenology of the abnormal movements was largely consistent with the definition of myorhythmia proposed by Masucci et al.: a low‐frequency, continuous or intermittent, relatively rhythmic movement that is present at rest and suppressed during sleep.4 This agreement led us to consider that the movements were at least a variant of myorhythmia. However, the frequency of the movements observed in the present case was too slow, compared with that of myorhythmia (generally 2–3 Hz).4 Moreover, it is atypical for myorhythmia that the mandibulo‐lingual muscles are exclusively affected, with sparing of the palate or limbs.
The etiology of myorhythmia includes brainstem stroke, cerebellar degeneration, and Whipple disease.5, 6 A previous study demonstrated that the SN and cerebellum were predominantly involved in autopsied cases of myorhythmia and suggested their role as generators of myorhythmia.4 Indeed, the present case exhibited bilateral MRI abnormalities in the SN, a region frequently affected by the JE virus,7 and we considered the lesion as the most likely cause of the abnormal movements. Other possible explanations for the movements include drug side effects and hypoxic brain damage. The patient had never been exposed to neuroleptics, and single‐pulse corticosteroid therapy or IVIg have the least possibility of causing a prolonged tremor. Hypoxic damage to the basal ganglia was also unlikely to have caused the movements, because the patient was intubated as soon as her breathing stopped. We confirmed the absence of anti‐NMDA (N‐methyl‐D‐aspartate receptor) receptor Ab in her CSF.
This intriguing case with unusually slow tremor broadens and challenges the classical definition of myorhythmia. The accumulation of similar cases is needed to clarify the etiology of this distinct type of tremor.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
T. Takeuchi: 1A, 1C, 3A
R.M.: 1A, 1C, 3A
Y.O.: 1C, 3B
T. Takasaki: 1C, 3B
N.Y.: 1C, 3B
K.S.: 1C, 3B
K.F.: 1C, 3B
Y. I.: 1B, 3B
R.K.: 1A, 1B, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported by Grants‐in‐Aid from the Research Committee of Dystonia, the Ministry of Health, Labor and Welfare of Japan.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
A video accompanying this article is available in the supporting information here.
Video. Symptoms at 46 days after onset of symptoms. Segment 1: The slow, repetitive tongue protrusion and jaw retraction are presented in a stereotyped manner. The right‐lower facial muscles are also involved. Notably, she exhibits a dull, mask‐like face. Segment 2: Flaccid paralysis is detected in all four limbs.
Acknowledgments
The authors thank Dr. Keiko Tanaka (Department of Neurology, Kanazawa Medical University, Ishikawa, Japan) for the anti‐NMDA receptor Ab test.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Solomon T, Dung NM, Kneen R, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000;68:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solomon T, Kneen R, Dung NM, et al. Poliomyelitis‐like illness due to Japanese encephalitis virus. Lancet 1998;351:1094–1097. [DOI] [PubMed] [Google Scholar]
- 3. Kalita J, Misra UK. Markedly severe dystonia in Japanese encephalitis. Mov Disord 2000;15:1168–1172. [DOI] [PubMed] [Google Scholar]
- 4. Masucci EF, Kurtzke JF, Saini N. Myorhythmia: a widespread movement disorder. Clinicopathological correlations. Brain 1984;107:53–79. [DOI] [PubMed] [Google Scholar]
- 5. Herrz E. The phenomenon of muscles cannot be fully rest. Clinical and cinematographic analysis of its characteristics and associated phenomena. J Psychol Neurol 1931;43:146–163. [Google Scholar]
- 6. Simpson DA, Wishnow R, Gargulinski RB, Pawlak AM. Oculofacial‐skeletal myorhythmia in central nervous system Whipple's disease: additional case and review of the literature. Mov Disord 1995;10:195–200. [DOI] [PubMed] [Google Scholar]
- 7. Kalita J, Misra UK, Pandey S, Dhole TN. A comparison of clinical and radiological findings in adults and children with Japanese encephalitis. Arch Neurol 2003;60:1760–1764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video. Symptoms at 46 days after onset of symptoms. Segment 1: The slow, repetitive tongue protrusion and jaw retraction are presented in a stereotyped manner. The right‐lower facial muscles are also involved. Notably, she exhibits a dull, mask‐like face. Segment 2: Flaccid paralysis is detected in all four limbs.


