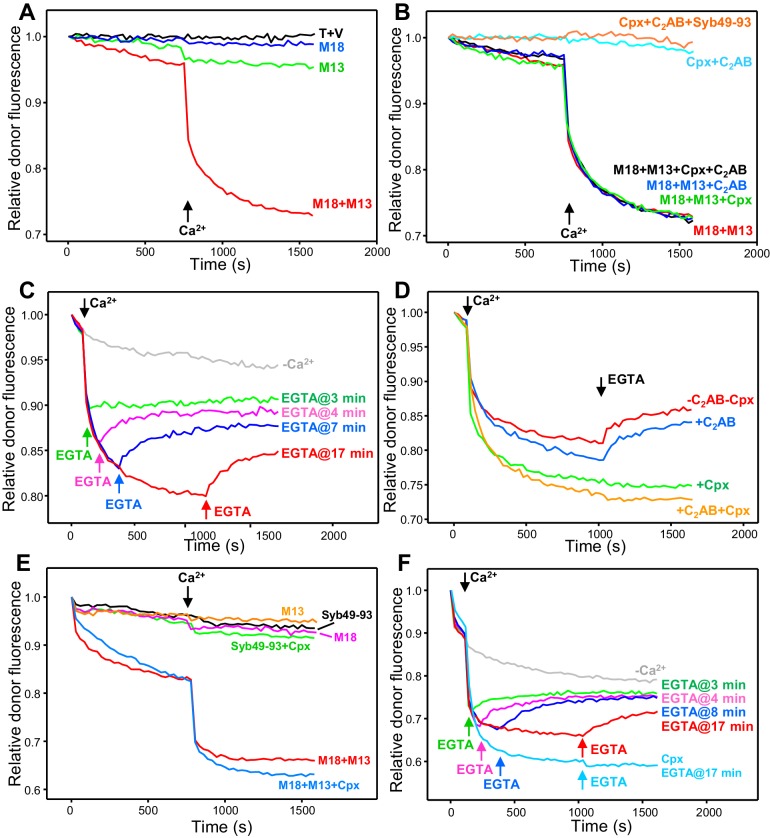
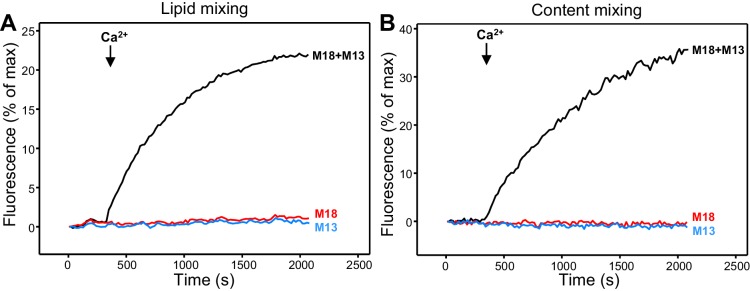
Figure 3. Influence of Munc18-1, Munc13-1 C1C2BMUNC2C, complexin-1 and synaptotagmin-1 on trans-SNARE complex assembly-disassembly in the presence of NSF-αSNAP.
(A,B) Kinetic assays monitoring trans-SNARE complex assembly between V- and T-liposomes (1:4 ratio) in the presence of NSF-αSNAP from the decrease in the donor fluorescence emission intensity. The experiments were performed in the absence of other proteins (T + V) or in the presence of different combinations of Munc18-1 (M18), Munc13-1 C1C2BMUNC2C (M13), complexin-1 (Cpx), synaptotagmin-1 C2AB and Syb49-93, as indicated by the colors. Experiments were started in 100 μM EGTA and Ca2+ (600 μM) was added after 750 s. (C) Analogous kinetic assays performed in the presence of Munc18-1, Munc13-1 C1C2BMUNC2C, NSF-αSNAP and 100 μM EGTA, but adding 240 μM Ca2+ at 2 min to stimulate trans-SNARE complex assembly and adding 500 μM EGTA at different times to chelate the Ca2+ and interrogate whether there is trans-SNARE complex disassembly. An experiment that was also started in 100 μM EGTA but without addition of Ca2+ or EGTA at later times (gray trace) is shown for comparison. (D) Experiments analogous to those of (C), with addition of 240 μM Ca2+ at 2 min and 500 μM EGTA at 17 min, performed in the absence or presence of complexin-1 and/or synaptotagmin-1 C2AB. (E) Kinetic assays monitoring trans-SNARE complex assembly between VSyt1- and T-liposomes (1:4 ratio) in the presence of NSF-αSNAP and different combinations of Munc18-1, Munc13-1 C1C2BMUNC2C, complexin-1 and Syb49-93, as indicated by the colors. Experiments were started in 100 μM EGTA and Ca2+ (600 μM) was added after 750 s. (F) Kinetic assays analogous to those of (E) performed in the presence of Munc18-1, Munc13-1 C1C2BMUNC2C, NSF-αSNAP and 100 μM EGTA, but adding 240 μM Ca2+ at 2 min to stimulate trans-SNARE complex assembly and adding 500 μM EGTA at different times to chelate the Ca2+ and interrogate whether there is trans-SNARE complex disassembly. An experiment that was also started in 100 μM EGTA but without addition of Ca2+ or EGTA at later times (gray trace) is shown for comparison. The light blue trace shows an additional experiment started in 100 μM EGTA in the presence of complexin-1, with addition of 240 μM Ca2+ at 2 min and 500 μM EGTA at 17 min. All experiments were performed in the presence of Mg2+ and ATP. For all traces shown in (A–F), fluorescence emission intensities were normalized with the intensity observed in the first point and corrected for the dilution caused by the addition of reagents.
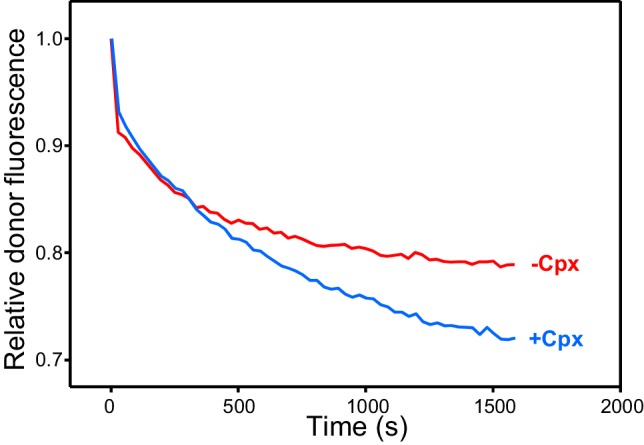
Figure 3—figure supplement 1. Complexin-1 increases the efficiency of Ca2+-independent trans-SNARE complex assembly between VSyt1- and T-liposomes in the presence of NSF-αSNAP.