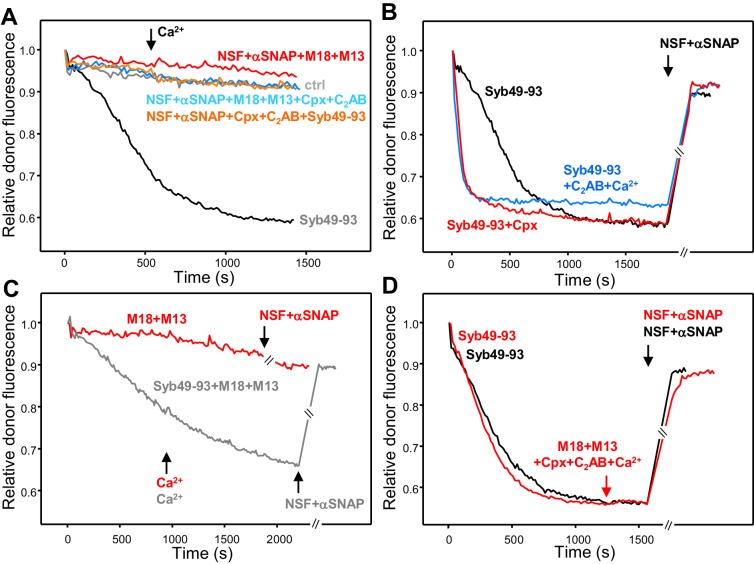
Figure 6. Munc18-1, Munc13-1 C1C2BMUNC2C, complexin-1 and synaptotagmin-1 C2AB do not decrease the overall amount of cis-SNARE complex disassembly caused by NSF-αSNAP.
(A) Kinetic assays monitoring changes in the donor fluorescence emission intensity due to cis-SNARE complex formation upon mixing V-liposomes containing Alexa488-synaptobrevin with an excess of TMR-labeled syntaxin-1 (2–253) and SNAP-25m in the presence of NSF-αSNAP with no additions (ctrl) (light gray trace) or with different combinations of Munc18-1 (M18), Munc13-1 C1C2BMUNC2C (M13), complexin-1 (Cpx) and synaptotagmin-1 C2AB as indicated. Ca2+ was added at 550 s. For comparison purposes, the dark gray trace shows a cis-SNARE complex assembly reaction performed in the presence of Syb49-93 and absence of NSF-αSNAP. (B) Kinetic assays of cis-SNARE complex assembly analogous to those of (A), but performed in the absence of NSF-αSNAP and the presence of Syb49-93 alone (black trace) or together with complexin-1 (Cpx) (red trace) or synaptotagmin-1 C2AB plus Ca2+ (blue trace). NSF-αSNAP were added when the reactions reached a plateau (black arrow) to monitor cis-SNARE complex disassembly. (C) Kinetic assays analogous to those in (B), but in the presence of Munc18-1 (M18) and Munc13-1 C1C2BMUNC2C (M13) without (red trace) or with (dark gray trace) Syb49-93. Ca2+ was added after 950 s. (D) Kinetic assays where cis-SNARE complex formation was initially catalyzed by Syb49-93 and, after reaching a plateau, Munc18-1, Munc13-1 C1C2BMUNC2C, complexin-1, synaptotagmin-1 C2AB and Ca2+ were added (red arrow); after five minutes, NSF-αSNAP were added to test for disassembly (red trace). The black trace shows a control experiment where the four proteins were not included before adding NSF-αSNAP. In the experiments shown in (C–D), we stopped monitoring the donor fluorescence intensity to add the reagents for disassembly, and a few minutes elapsed until we started to monitor the reaction again (indicated by the double slanted bars on the traces and on the x axis). For all traces of (A–D), fluorescence emission intensities were normalized with the intensity observed in the first point and corrected for the dilution caused by the addition of reagents.
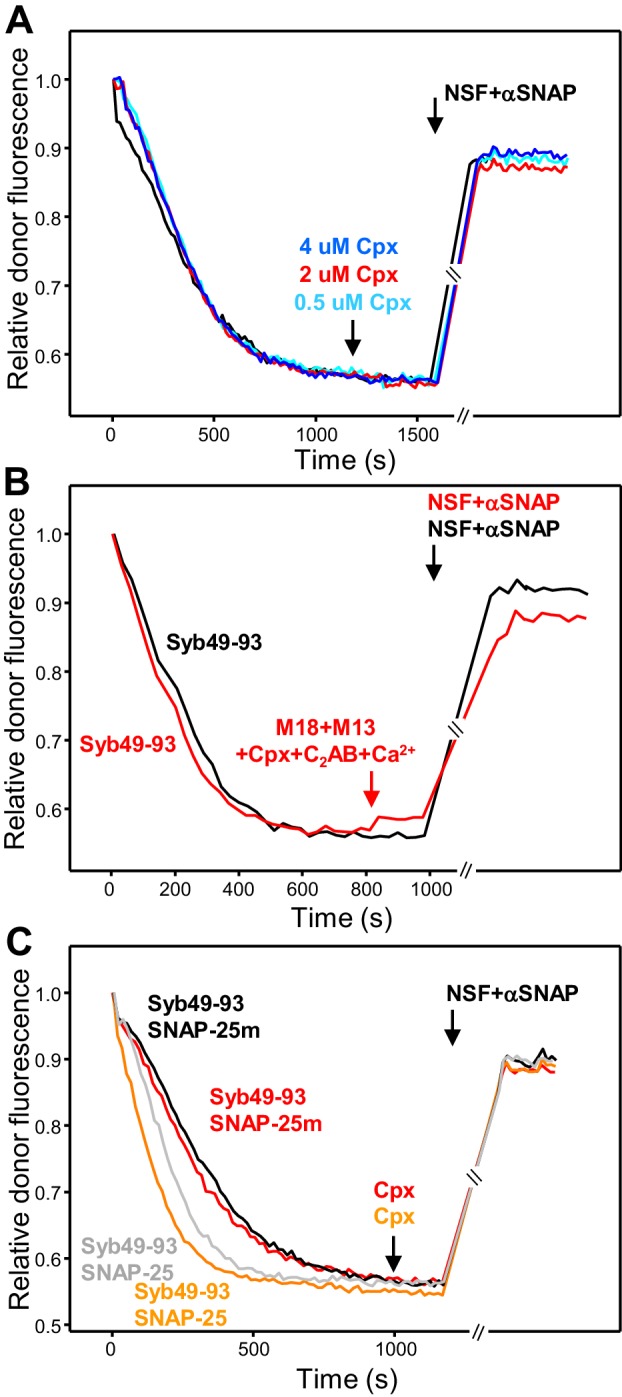
Figure 6—figure supplement 1. Munc18-1, Munc13-1 C1C2BMUNC2C, complexin-1 and synaptotagmin-1 C2AB do not protect cis-SNARE complexes against disassembly by NSF-αSNAP.