Figure 7.
MyoII Polarity Stiffens the Tissue and is Required for Wing Disc Shape Maintenance via Dia-Dependent Actin Polymerization
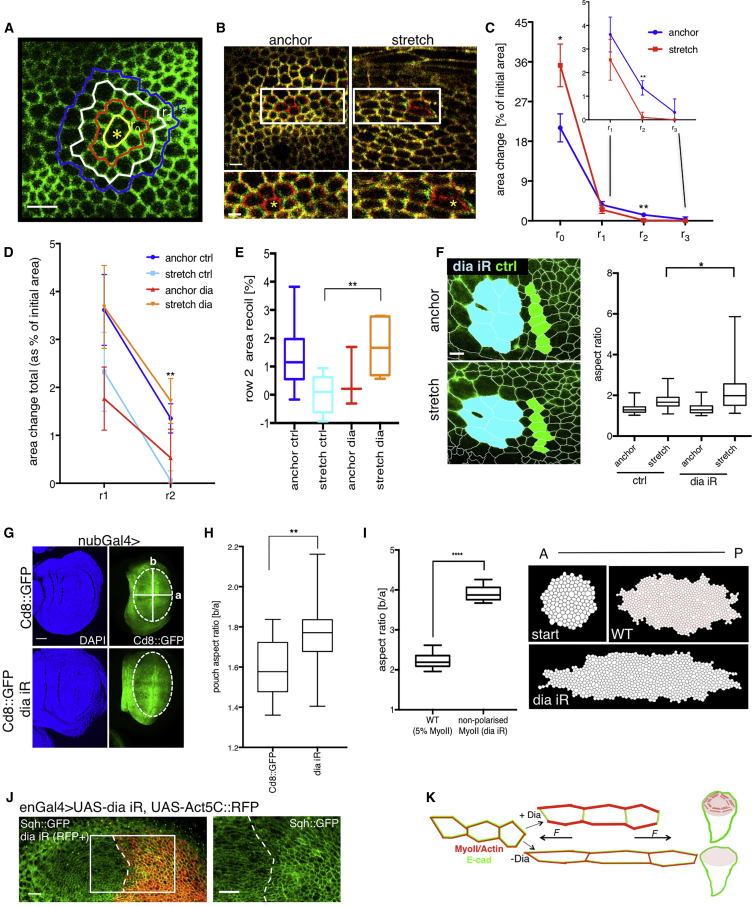
(A) Image depicting injury site (yellow asterisk) and selection of cell rows used for tissue recoil propagation measurement; r: row.
(B) An overlay of anchored and stretched discs prior to ablation (red) and 15.44 s post ablation (green). Inset zooms in on the area next to ablation site (marked with yellow asterisk).
(C) Recoil area (% increase from pre-ablation area) for row 0, row 1, row 2, and row 3 in stretched and anchored discs. Data is plotted as mean ± S.E.M.; n = 8–13 discs per condition.
(D) Recoil area (% increase from pre-ablation area) for row 1 and row 2 in stretched and anchored discs for wild-type (control) and dia-RNAi discs. Data is plotted as mean ± S.E.M.; n = 8–13 discs per condition.
(E) Box plot showing recoil area (% increase from pre-ablation area) for row 2 (same data as D). Median represented by horizontal line; 75th and 25th percentiles are represented by top and bottom of the boxes respectively. Statistical test compares wild-type (control) stretch with dia-RNAi stretch discs.
(F) Discs expressing E-cad::GFP and hhGal4>dia-RNAi (KK) subjected to stretch; selection of control (green) and dia-RNAi (cyan) for cell deformation analysis. Box plot showing distribution of cell aspect ratio in anchored and stretched (30 min) wing disc for control (E-cad::GFP expressing) and dia-RNAi cells; median represented by horizontal line; 75th and 25th percentiles are represented by top and bottom of the boxes respectively; n = 3 wing discs.
(G) Discs expressing nubGal4 and Cd8::GFP (control) or dia-RNAi (KK) labeled with DAPI; ellipse is fitted into the pouch region (dotted line) and aspect ratio determined as long ellipse axis (b) divided by short ellipse axis (a) (white lines).
(H) Box plot showing distribution of wing disc pouch aspect ratios in conditions described in (G); n = 19 (ctrl) and 16 (dia iR) wing pouches; horizontal line indicates median.
(I) Influence of myosin increase upon tissue stretching to emergent tissue shape. Start: Initial configuration of the monolayer to be stretched in horizontal direction (A-P axis). WT: Configuration of the monolayer after 12 h simulation with 5% increase in tension levels on horizontal junctions that are within 30 degrees of the stretch axis. The junctions with increased tension are labeled in red. dia iR: Configuration of the monolayer upon 12 h simulation with no myosin response. Quantification of the aspect ratio of the central region of the monolayer (displayed in white). Single cells detached from the clone are excluded from the analysis. Mean and standard deviation of 10 simulation for each case are displayed. No myosin response case is significantly different from 5% myosin increase (two-tailed t test; p value 2.55E−11).
(J) Endogenous Sqh::GFP polarization in enGal4> dia iR, UAS-Act5C::RFP discs. Sqh::GFP does not polarize in dia-RNAi side of disc.
(K) Model of diaphanous-myosin force buffering pathway. Tissue (E-cad marks cell junctions in green) subjected to mechanical stretching polarizes MyoII and Actin (in red) in Dia-dependent manner. This mechanism is required to limit tension-induced cell deformation and thus maintain wing disc pouch shape.
∗ p < 0.05,∗∗ p < 0.01 and **** p < 0.0001 with t test unless otherwise stated. Scale bars, 5 μm (B inset and F), 10 μm (A, B and J), 30 μm (G).