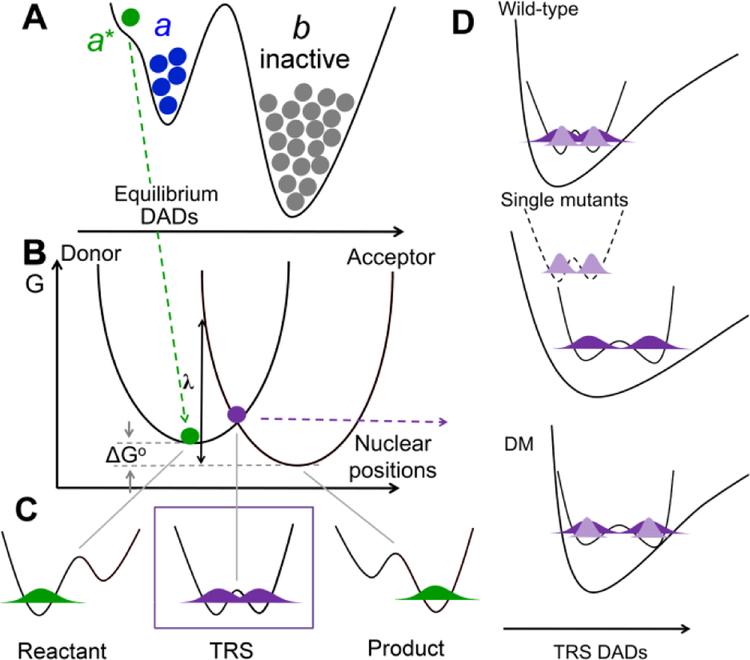
Figure 5.
Rate constants for enzyme catalyzed nonadiabatic hydrogen transfer can be formalized as kobs = Keq • kPCET.9, 28, 33 (A) Keq (<< 1) represents a stochastic ground state search through inactive conformations (b) to reach catalytically active E-S complexes (a conformer). Subsequent thermal sampling further reduces the distance between the H-donor and acceptor as seen in the pre-tunneling a* conformer. (B-D) illustrate contributions from kPCET: (B) Heavy atom protein motions produce transiently degenerate energies for the reactant and product wells, a prerequisite for wave function overlap at the tunneling ready state (TRS) (C). (D) The effective potential along the DAD sampling coordinate varies, starting with ΔEa ≅ 0 for native enzyme (top), becoming ΔEa >>0 when the DAD is elongated following single mutations (middle) and finally arriving at the catastrophic DM scenario in which the DAD is elongated, but coupled with a rigid DAD sampling potential (bottom). In Frame D, light purple refers to the shorter wave function distribution for deuterium, and dark purple represents the more distributive protium wave function.