Abstract
Background:
Social and environmental stressors, may modify associations between environmental pollutants and asthma symptoms. We examined if neighborhood asthma prevalence (higher: HAPN vs. lower: LAPN), a surrogate for underlying risk factors for asthma, modified the relationship between pollutants and urgent asthma visits.
Methods:
Through zip code, home addresses were linked to New York City Community Air Survey’s land use regression model for street-level, annual average nitrogen dioxide (NO2), particulate matter (PM2.5), elemental carbon (EC); summer average ozone (O3); winter average sulfur dioxide (SO2) concentrations. Poisson regression models were fit to estimate the association (prevalence ratio, PR) between pollutant exposures and seeking urgent asthma care.
Results:
All pollutants, except O3 were higher in HAPN than LAPN (P<0.01) Neighborhood asthma prevalence modified the relationship between pollutants and urgent asthma (P-interaction<0.01, for NO2 and SO3). Associations between pollutants and urgent asthma were observed only in LAPN (NO2: PR=1.38, P=0.01; SO3: PR=1.85, P=0.04). No association was observed between pollutants and urgent asthma among children in HAPN (P>0.05).
Conclusions:
Relationships between modeled street-level pollutants and urgent asthma were stronger in LAPN compared to HAPN. Social stressors that may be more prevalent in HAPN than LAPN, could play a greater role in asthma exacerbations in HAPN versus pollutant exposure alone.
Introduction
Pediatric asthma is a chronic, heterogeneous disease of airflow limitation that can manifest with attacks commonly triggered by environmental exposures.(1) Exposure to environmental air pollution is known to trigger asthma attacks leading to unscheduled, urgent medical visits for asthma. Numerous studies have demonstrated increases in emergency department (ED) visits and hospitalizations in association with increasing concentrations of outdoor ambient pollutants.(2–8) Furthermore, increased concentrations of air pollution in urban environments has been deemed one of the contributors to asthma disparities between urban and non-urban populations.(9–11) However, spatial variation in concentration of pollutants exist within a community because of differences in point sources(12, 13) and traffic patterns;(14, 15) which may place children that live in neighbouring areas at varying levels of risk for asthma attacks.
Similar to ambient pollutant concentrations, the prevalence of asthma is not evenly distributed throughout some urban communities.(16, 17) For example, asthma prevalence in New York City (NYC) among 4–5 year old children has been shown to vary from 3–19% across adjacent neighborhoods.(17) In the NYC Neighborhood Asthma and Allergy Study (NYCNAAS), an asthma case-control study of children from middle income families, asthmatic children living in neighborhoods with higher asthma prevalence (HAPN) made more urgent, unscheduled medical visits for asthma compared to asthmatic children living in lower asthma prevalence neighborhoods (LAPN). However, indicators of asthma severity (e.g. lung function and airway inflammation) did not differ among asthmatic children living in the HAPN as compared with those in LAPN.(18) This suggests that neighborhood-level risk factors, other than asthma severity and access to health care, may contribute to the disparity in urgent medical visits between neighborhoods within a small geographic region.
Several studies have demonstrated that social and environmental stressors, including parental stress, (19, 20) chronic childhood stress (21) and exposure to violence (22) are associated with a stronger relationship between environmental pollutants and asthma development and symptoms. In an adult population in Northern California, Blanc and colleagues observed that neighborhood-level SES predicted different asthma outcomes in low versus suburban SES neighborhoods.(23) A recent study in Atlanta, Georgia, demonstrated stronger associations between neighborhood-level air pollution and ED visits for asthma among children living in communities with extremely low neighborhood socioeconomic status (SES) compared to higher SES neighborhoods.(24) While this study is very informative, neighborhood-level SES is only one element of the contextual community level characteristics that contributes to differential asthma risk.(25) The prevalence of asthma within a neighborhood, as assessed in the NYCNAAS, may be a better indicator that encompasses measured and unmeasured built, social and environmental risk factors within each community that contribute to increased risk of asthma development and morbidity.
Our objective was to determine if the relationship between ambient pollutants and unscheduled, urgent visits for asthma (primary physician or ED) varied between NYC neighborhoods with high versus low asthma prevalence. We examined this relationship in a cohort of 7–8 year old children with asthma in NYC specifically recruited to examine neighborhood differences in environmental exposures and asthma morbidity. Due to higher concentrations of pollutants in neighborhoods with higher asthma prevalence, we hypothesized that the relationship between exposure to pollutants and urgent medical visits for asthma would be greater for children living in HAPN.
Methods
Study participants
Parents of children ages 7–8 years were recruited through a middle-income insurance provider for the NYC Neighborhood Asthma and Allergy case-control study (NYCNAAS), as previously described in detail.(18, 26) Briefly, neighborhoods were selected throughout the Bronx, Brooklyn, Queens, and Manhattan (Figure 1) based on zip code level asthma prevalence for 4–5 year olds as reported by the Department of Health and Mental Hygiene (DOHMH).(27) Asthma classification was based on a positive response to at least one of two questions completed by the child’s physician: 1) an ICD-9 diagnosis code for asthma (493.XX) or 2) an indication of the child having “ever been diagnosed” with asthma. NYC DOHMH published United Hospital Fund (UHF) neighborhood level data for asthma prevalence among 4–5 year olds. These UHF geographies correspond to commonly described neighborhoods (e.g., East Harlem, the Upper East Side) and are a collection of zip codes. We used the reported prevalence for a UHF neighborhood as the prevalence for each of the zip codes in that neighborhood. The zip codes used both by NYC DOHMH and our study correspond to the residence of the child, not the school. Neighborhoods were stratified by zip code into higher asthma prevalence neighborhoods (HAPN, 11–18%) and lower asthma prevalence neighborhoods (LAPN, 3–9%). Children were recruited simultaneously from HAPN and LAPN throughout 2008–2011. Recruitment was conducted through a middle-income insurance provider to limit the potential for confounding by lack of access to insurance-based medical care. The neighborhood asthma prevalence served as a proxy encompassing measured and unmeasured built, social and environmental risk factors for high vs. low risk urban asthma. This study was approved by the Columbia University Institutional Review Board and informed consent was obtained from all participants.
Figure 1:
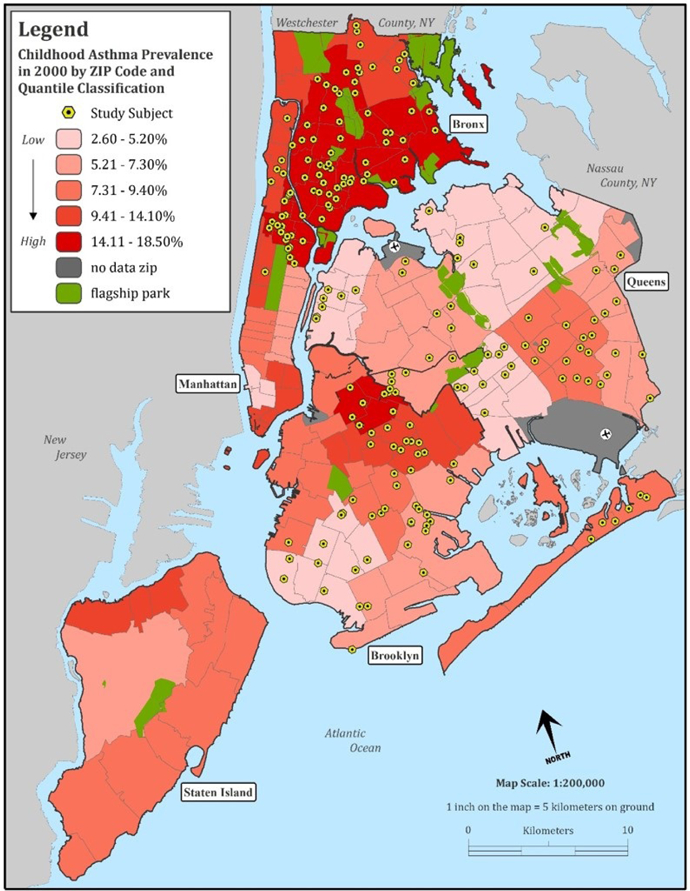
New York City map illustrating asthma prevalence by neighborhood and residential locations for children that were recruited for this study.
Asthma definitions
Only children classified as having asthma were included in the current analysis. Asthma was defined, as previously published,(18, 26) by a report of at least one of the following during the preceding 12 months: 1) wheeze, 2) awakening at night by cough without having a cold, 3) wheeze with exercise, or 4) report of asthma medication use. Exercise induced wheeze was defined by responding ‘yes’ to: 1) wheezing after running or active play and/or 2) child’s chest sounding wheezy during or after exercise. Because of the relatively low frequency of ED visits for asthma in this middle income population with access to private insurance, we considered any unscheduled urgent visits for asthma as the outcome of interest. Urgent asthma visits were obtained by questionnaire and defined as any unscheduled visit to the doctor in a hurry or to the ED due to breathing difficulties in the past 12 months. This broad definition allowed us to capture a range of asthma exacerbations that required urgent medical attention as opposed to ED visits alone.
Air pollution exposure assessment
Children’s home and school addresses were linked through zip code to the NYC Community Air Survey’s (NYCCAS) land use regression model for street-level ambient pollutant concentration.(28) NYCCAS collects pollution measures annually, during each meteorological season, using stationary site monitors, mounted 10–12 feet from the ground at 100 locations throughout NYC. Data were analyzed for annual average concentrations of nitrogen dioxide (NO2), fine particulate matter (PM2.5), elemental carbon (EC), summer average ozone (O3) (June – August), and winter average sulfur dioxide (SO2) (December – February). Annual average pollutant concentrations were matched to NYCCAS data published during the study recruitment period. For this cross-sectional analysis, the use of annual average exposure reduced the influence of seasonal fluctuations in individual pollutants across the concurrent 1-year asthma outcome observation period. O3 and SO2 concentrations were reported by NYCCAS only in the summer and winter respectively due to high seasonal peaks and relatively low concentrations outside of those seasons. Pollutant concentrations were very highly correlated between home and school addresses for all pollutants (rspearman >0.98, P<0.01) as most children attended school in the neighborhood where they lived. Therefore, for the current analysis we used concentrations of pollutants at home addresses only.
Neighborhood built environment assessment
Children’s home addresses were geocoded using the NYC Department of City Planning GeoSupport application. United States census data from 2000 was applied using geographic information systems (GIS) technology to determine the demographic (e.g. population, race, housing and education) characteristics of the community. Census data were apportioned according to the percentage of land area within a 0.5 km buffer surrounding the child’s home address. Pollution point source variables were determined using methodology that was developed by Maatay and colleagues.(29) Truck route and street density were calculated as miles of roadway per km2 within the 0.5 km buffer.
Statistical Analysis
Chi-square tests (for categorical data) and T-tests (for continuous data) were used to compare demographic and clinical outcomes between HAPN and LAPN. Spearman correlations were used to compare pollutant concentrations to each other and Kruskal-Wallis tests were used to compare pollutant concentrations between HAPN and LAPN.
Modified Poisson regression models with robust standard errors were fit to determine the prevalence ratio (PR) of seeking urgent asthma care in the last 12 months for a given increase in pollutant concentration. Models were clustered on zip code of residence for children who lived in the same zip code. Results represent a PR per 1 unit increase in pollutant concentration. Final models were built based on the overall NO2 model. Variables were selected from a pool of common covariates that are widely published in pediatric asthma research as having been associated with NO2 exposure or urgent medical visits for asthma. Additional covariates were included based on differences that were observed between low and high asthma prevalence neighborhoods (eg, apartment type). In prior work we demonstrated that the floor a child lives on within a building can directly influence indoor concentrations of street-level pollutants (30). To account for social and family stress we adjusted for maternal hardship that was determined if the parent responded yes to having concerns regarding food, housing, gas or electricity, clothing, or medical treatment in the preceding 12 months. Preventive asthma medication (e.g. inhaled corticosteroids) was selected as an indicator of asthma severity. Covariates remained in the model if the addition of the new variable resulted in at least one of the following: 1) an change in the parameter estimate for NO2 being associated with urgent medical visits of > 10%, 2) a P-value <0.05 for the covariate, or 3) a decrease in the Quasiliklihood under the Independence model Criterion (QIC) for the overall model. All final models included sex, race, Latino ethnicity, preventive asthma medication use, exercise-induced wheeze, environmental tobacco smoke exposure, maternal asthma, parental income, residential building type, apartment floor height, maternal hardship, truck route density and neighborhood asthma prevalence. In separate models, an interaction term was tested to determine if neighborhood asthma prevalence modified the association between pollutants and urgent asthma care. Models then were stratified by neighborhood asthma prevalence. In secondary analysis models were further stratified by dust mite allergen (derf1), because of the potentiating effect of dust mite allergen and air pollutants on respiratory outcomes (31). Additionally, we stratified by floor of the child’s residence within the apartment, to assess if children that lived closer to street level (floors 0–2) had a stronger association between street-level modeled pollutants and urgent asthma compared to children that lived on higher floors. All statistical analyses were performed using SAS 9.4.
Results
Participant characteristics
Demographic characteristics for the 190 asthmatic children included in this analysis are displayed in Table 1. More children in HAPN lived in apartment buildings (P<0.01) and on higher floors (P<0.01) compared to children in LAPN. As previously reported,(32) more asthmatic children in HAPN had exercise-induced wheeze (P=0.02) and urgent asthma visits (P=0.04) compared to children in LAPN.
Table 1:
Participant characteristics for n=190 children with asthma that were included in this study.
| LAPN (n=91) | HAPN (n=99) | P-valuea | |
|---|---|---|---|
| Demographics | |||
| Female sex, n (%) | 36 (40%) | 46 (46%) | 0.34 |
| Ethnicity/race, n (%) | |||
| Latino | 21 (23%) | 43(43%) | <0.01 |
| Black | 41 (45%) | 54 (55%) | 0.19 |
| White | 18 (20%) | 8 (8%) | 0.02 |
| Asian | 15 (16%) | 2 (2%) | <0.01 |
| Other/mixed race | 14 (15%) | 33 (33%) | <0.01 |
| Obese, n (%)b | 20 (22%) | 20 (21%) | 0.82 |
| Maternal asthma, n (%) | 21 (23%) | 28 (28%) | 0.41 |
| Paternal asthma, n (%) | 17 (19%) | 16 (16%) | 0.65 |
| Parental income, mean (SD) | $56,484 (26,900) | $50,328 (24,660) | 0.10 |
| Season of home visit, n (%) | 0.48 | ||
| Jan-Mar | 21 (23%) | 22 (22%) | |
| Apr-Jun | 27 (30%) | 37 (37%) | |
| Jul-Sep | 23 (25%) | 26 (26%) | |
| Oct-Dec | 20 (22%) | 14 (14%) | |
| Building type, n (%) | <0.01 | ||
| single/multi family | 56 (62%) | 22 (22%) | |
| apartment | 35 (38%) | 77 (78%) | |
| Floor height, mean (SD)c | 2.8 (2.5) | 5.3 (5.9) | <0.01 |
| Environmental tobacco smoke, n (%) | 14 (15%) | 25 (25%) | 0.09 |
| Material hardship, n (%) | 25 (27%) | 30 (30%) | 0.67 |
| Clinical Outcomes | |||
| Preventive medication, n (%) | 56 (62%) | 62 (63%) | 0.88 |
| Exercise-induced wheeze, n (%) | 33 (36%) | 53 (54%) | 0.02 |
| Urgent asthma visit, n (%) | 18 (20%) | 33 (33%) | 0.04 |
| ED asthma visit, n (%) | 14 (7%) | 21 (11%) | 0.30 |
| FEV1/FVC, mean (SD)d | 0.87 (0.05) | 0.87 (0.06) | 0.91 |
| FeNO, GM (SD)e | 11.5 (2.04) | 11.1 (2.25) | 0.75 |
| Seroatopic, n (%)f | 54 (62%) | 59 (63%) | 0.92 |
| Dust mite exposure (µg/g), GM (SD) | 0.06 (4.12) | 0.05 (3.29) | 0.22 |
| Neighborhood Characteristics | |||
| Race/Ethnicity, mean percent (SD) | |||
| Black | 40% (36) | 48% (26) | 0.07 |
| Latino | 18% (14) | 42% (23) | <0.01 |
| White | 35% (27) | 22% (18) | <0.01 |
| Poverty, mean percent (SD) | 18% (10) | 34% (13) | <0.01 |
| Public assistance, mean percent (SD) | 6% (4) | 15% (7) | <0.01 |
| Crowded housing, mean percent (SD) | 15% (7) | 18% (7) | <0.01 |
| High school educated, mean percent (SD)g | 72% (9) | 60% (12) | <0.01 |
| Single mother, mean percent (SD) | 11% (7) | 21% (7) | <0.01 |
| Street density, km2 (SD) | 17.4 (2.9) | 17.4 (3.2) | 0.98 |
| Truck route density, km2 (SD) | 1.8 (1.1) | 2.3 (1.4) | 0.02 |
| Air pollution point source, mean percent (SD)h | 5% (13) | 12% (20) | <0.01 |
Represents comparison between LAPN and HAPN using chi-square test with the exception of non-categorical variables: floor height, parental income, FEV1/FVC, FeNO, dust mite exposure and Neighborhood Characteristics (T-test).
Defined as BMI ≥ 95th percentile for age and sex
Missing n=2 from HAPN
Missing n=18 from LAPN and n=10 from HAPN.
Missing n=7 from LAPN and n=8 from HAPN.
Seroatopy defined as specific IgE > 0.35 IU/mL to any one of the following allergens: dust mite (d2), cockroach (i6), mouse (e72), cat (e1), dog (e5), ragweed (w1), tree pollen (tx8) and grass (gx2). Missing n=4 from LAPN and n=5 from HAPN.
Defined as percent of the population 25 years and older that graduated from high school.
BMI: body mass index; LAPN: lower asthma prevalence neighborhood; HAPN: higher asthma prevalence neighborhood; FeNO fractional exhaled nitric oxide; GM: geometric mean; SD: standard deviation; FEV1: forced expiratory volume in 1 second; FVC:forced vital capacity.
Percent of coverage within a 0.5-mile buffer surrounding participants’ address identified as a stationary point source of pollution by the National Emissions Inventory.
Based on US census data from 2000, HAPN had a greater percentage of the population that were self-identified as black (% (SE): 48% (2.6)) and Latino (42% (2.3)) compared to LAPN (black: 40% (3.8), P=0.07; Latino: 18% (1.4), P<0.01) while LAPN had a greater percentage of white families (35% (2.8)) compared to HAPN (22% (1.8), P<0.01) (Table 1). HAPN vs. LAPN had a greater percentage of the population living below the federal poverty level (34% (1.3) vs. 18% (1.1), P<0.01), on public assistance (15% (0.7) vs. 6% (0.4), P<0.01), living in crowded housing (18% (0.8) vs. 15% (0.8), P<0.01), and had households with single mothers (21% (0.7) vs. 11% (0.8), P<0.01) (Table 1). Additionally, a greater percentage of the adult population in LAPN had a high school degree or higher compared to HAPN (72% (1.0) vs. 60% (1.0), P<0.01) (Table 1).
Air pollution concentrations
Street level modelled concentrations of NO2, PM2.5, EC and SO2 were all highly correlated (rspearman >0.66, P<0.01) (Supplemental Figure S1), while O3 concentrations were inversely correlated with the other pollutants (rspearman >−0.54, P<0.01) (Supplemental Figure S1). As anticipated based on prior NYCAAS reports,(33) annual average concentrations of NO2, PM2.5, EC and SO2 were higher in HAPN than in LAPN (P<0.01) (Supplemental Figure S2). O3 was higher in LAPN (P<0.01) (Supplemental Figure S2). The percent of land coverage by point sources of air pollution within a 0.5 km buffer was greater near HAPN homes (mean (SD): 12% (20)) compared to LAPN homes (5.5% (13), P<0.01). Also, truck route density was greater near HAPN homes (2.3 km2 (1.4)) compared to LAPN homes (1.8 km2 (1.1), P=0.02). Yet, there was no difference in street density between HAPN (17 km2 (3.2)) and LAPN (17 km2 (2.9), P=0.98).
Relationship between pollutants and urgent asthma visits
Overall, higher mean concentrations (±SD) of NO2 were observed among children that reported urgent asthma visits (25.8 ppb (2.98)) compared to children that reported no urgent asthma visits (24.1 ppb (3.41), P<0.01). Similarly, concentrations of PM2.5 and EC were higher among children that reported urgent asthma visits (PM2.5: 11.1 µg/m3 (0.73); EC: 1.15 µg/m3 (0.16)) compared to children with no urgent asthma visits (PM2.5: 10.7 µg/m3 (0.85); EC: 1.07 µg/m3 (0.18), P<0.01 for both). In multivariable regression models higher exposure to pollutants was associated with greater prevalence (PR) of urgent asthma visits (NO2: PR=1.11, 95% CI [1.01, 1.22]; Table 2).
Table 2:
Modified Poisson regression models demonstrating the prevalence ratio (PR) of seeking urgent asthma care in the last 12 months for a given increase in pollutant concentration. Overall sample and stratified analysis by neighborhood asthma prevalence are displayed. Neighborhood asthma prevalence modified the association between annual average modeled ambient pollutant concentration at the street-level and urgent asthma care.
| Overall Sample (n=190) | LAPN (n=91) | HAPN (n=99) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | P | PR | 95% CI | P | PR | 95% CI | P | P-interaction | |
| NO2 | 1.13 | 1.01, 1.26 | 0.04 | 1.60 | 1.20, 2.14 | <0.01 | 1.07 | 0.95, 1.21 | 0.24 | <0.01 |
| PM2.5 | 1.50 | 0.98, 2.30 | 0.06 | 3.58 | 1.26, 10.1 | 0.02 | 1.16 | 0.72, 1.87 | 0.55 | <0.01 |
| EC | 1.02 | 1.00, 1.04 | 0.05 | 1.02 | 0.99, 1.06 | 0.15 | 1.01 | 0.98, 1.04 | 0.64 | - |
| O3 | 0.92 | 0.79, 1.07 | 0.27 | 0.56 | 0.36, 0.87 | <0.01 | 0.96 | 0.84, 1.13 | 0.75 | 0.03 |
| SO2 | 0.98 | 0.83, 1.17 | 0.88 | 2.59 | 1.55, 4.34 | <0.01 | 0.94 | 0.82, 1.12 | 0.58 | <0.01 |
All models adjusted for sex, black race, Latino ethnicity, white/Asian/other/mixed race, preventive asthma medication use, exercise-induced wheeze, environmental tobacco smoke exposure, maternal asthma, parental income, building type, apartment unit floor height, maternal hardship, truck route density and neighborhood (only in non-stratified models). P-interaction represents the P value for the interaction term (pollutant x neighborhood).
LAPN: lower asthma prevalence neighborhood; HAPN: higher asthma prevalence neighborhood; PR: prevalence ratio; CI: confidence interval; NO2: nitrogendioxide; PM2.5: particulate matter <2.5 microns; EC: elemental carbon; O3: ground level ozone; SO2: sulfur dioxide
Effect modification by neighborhood asthma prevalence
An interaction term was introduced to all models and demonstrated that neighborhood asthma prevalence modified the association between pollutants and urgent asthma visit (NO2: Pinteraction<0.01, O3: Pinteraction=0.03, and SO2: Pinteraction<0.01) (Table 2). Findings were only observed among children in LAPN where higher exposure to pollutants was associated with a greater prevalence ratio of urgent asthma visits (NO2: PR=1.38, 95% CI [1.08, 1.76] and SO3: PR=1.85, 95% CI [1.04, 3.29], Table 2, Figure 2). An inverse association was observed between O3 and urgent asthma (PR=0.58, 95% CI [0.38, 0.89], Table 2, Figure 2); however, when this model was further controlled for summer NO2 concentration to account for the known scavenger effect of O3, the association was attenuated (PR=1.20, 95% CI [0.95, 1.51], P=0.34). No significant associations were observed between pollutants and urgent asthma visits among children in HAPN (P>0.05) (Table 2, Figure 2). Maternal hardship was a significant covariate (P<0.05) in all of the HAPN models but none of the LAPN models.
Figure 2:
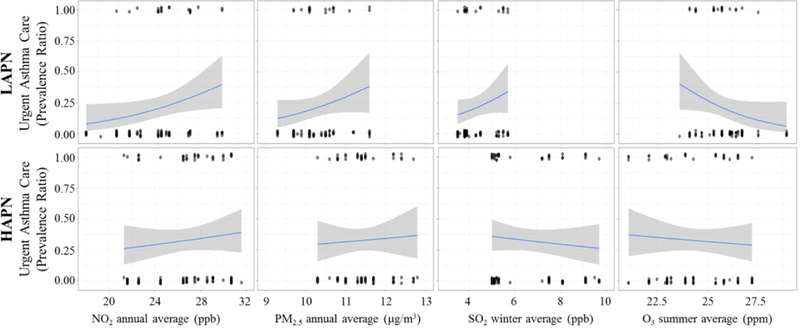
Prevalence ratio of seeking urgent asthma care (y-axis) for a given increase in pollutant concentration (x-axis) stratified by lower asthma prevalence neighborhoods (LAPN, top panels) versus higher asthma prevalence neighborhoods (HAPN, bottom panels). Street level ambient pollution is associated with increased prevalence of urgent asthma care among children in LAPN (n=90) and not HAPN (n=99), with the exception of O3.
Secondary analysis
Previous studies have demonstrated a potentiating effect of dust mite allergen and air pollutants on respiratory outcomes(31) and previously we demonstrated greater dust mite allergen concentrations in LAPNs(18), therefore we examined if the relationship between pollutants and urgent asthma differed by exposure to dust mite allergen (derf1). In secondary analysis in LAPN we observed a greater impact of pollutants (NO2, PM2.5, O3, and SO2) on urgent asthma visits among children with no dust mite allergen detected in house dust compared to children with detectible dust mite allergen (Supplemental Table S1).
To assess if children in HAPN that lived closer to street level (floors 0–2) had a stronger association between street-level modeled pollutants and urgent asthma compared to children that lived on higher floors we stratified analysis by apartment unit floor. Again we observed no significant association between pollutants and urgent asthma visits among children in HAPN; however, there was a non-significant trend towards a positive association between NO2 and urgent asthma visits among children in HAPN that lived on floors 0–2 (PR=1.42, 95% CI [0.92, 2.20], P=0.11, Supplemental Table S2).
Our case definition of asthma was intentionally more sensitive; however, this diminishes its specificity. To confirm the robustness of our findings, we conducted sensitivity analysis for which asthma was defined as having a report of wheeze-related symptoms ≥ 4 times or sleep disturbance ≥ 1 time per week in the past 12 months. The magnitude of our parameter estimates was reduced, possibly due to reduced power with the smaller sample size (n=78 asthmatics), all results remained statistically significant (Supplemental Table S3).
Discussion:
In our cohort of 7–8 year old children with asthma, from middle-income families throughout NYC, both the frequency of urgent asthma visits and the concentrations of ambient pollutants were higher for children living in the neighborhoods with higher as compare with lower asthma prevalence. However, despite this and contrary to our hypothesis, neighborhood-level outdoor pollutant concentrations were associated with increased urgent asthma care only among children living in LAPN. The lack of association between outdoor neighborhood pollution and urgent asthma visits in HAPN could suggest that additional unmeasured environmental, biological, and social factors may have more influence on urgent asthma visits in high risk children from middle income families living in HAPN compared to LAPN.
Recently, O’Lenick and colleagues conducted a similar population based study in Atlanta, Georgia using hospital records of pediatric ED visits for asthma and simulated pollution estimates from stationary monitors throughout the city.(24) The authors reported stronger associations between air pollution and asthma ED visits among children living in extremely poor communities (≥20% of population below federal poverty level) compared to less poor communities (<20% below poverty level). Similarly, in a population based adult study in NYC, Kheirbek and colleagues observed higher air pollution attributed asthma ED visits in high poverty neighborhoods compared to low poverty neighborhoods.(13) Our study was not designed to assess differences in pollution and urgent asthma by neighborhood poverty level alone; rather, we used neighborhood asthma prevalence as a proxy for measured and unmeasured built, social and environmental factors, including poverty, that contribute to higher vs. lower asthma risk. Interestingly we observed a paradoxical findings, whereby the observed relationship between pollution exposure and urgent asthma visits was strongest among children living in less impoverished and lower asthma risk neighborhoods.
A distinct difference between the aforementioned studies and ours is that we sampled only children from middle income families in both high and low risk asthma neighborhoods. The children recruited from HAPN lived in communities that had a greater percentage of residents that lived below the federal poverty level and were on public assistance compared to children from LAPN. However, household incomes and access to health care (private insurance) did not significantly differ between the two groups of children in our study. Thus, the children from our study that lived in HAPN did not necessarily represent their neighborhood characteristics. Neighborhood characteristics and social influences (e.g. stress and violence)(34) that may be more prevalent in HAPN than LAPN, could play a greater role in asthma exacerbations and urgent asthma care in middle income families living in these neighborhoods versus exposure to pollutants alone. This is supported by the observation that maternal hardship was a significant covariate (P<0.05) in all of the HAPN models but none of the LAPN models (data not shown). In addition, population based studies may be subject to ecological fallacy whereby inferences about individuals are presumed from inferences for the group that the individuals come from. A strength of our study is that we assessed urgent asthma outcomes on the individual level.
Another explanation for the observed association between air pollution and urgent asthma care only in LAPN could be due to biological interactions between pollutants and allergens. Previously we reported that homes in LAPN had higher concentrations of house dust mite allergen, while HAPN homes had higher concentrations of cockroach and mouse allergen.(18) In one study of adults with asthma, inhalation of NO2 followed by inhalation challenge with house-dust mite allergen triggered greater reduction in forced expiratory volume in 1 second (FEV1) than challenge with house-dust mite allergen alone. These findings suggest that pollutants can potentiate the impact of dust mite allergen exposure on airway disease.(31) However, we observed the opposite in our cohort. In children living in LAPN the relationship between pollutants and urgent asthma was attenuated among those with detectible dust mite allergen exposure compared to children with no dust mite. This contradictory finding warrants further investigation in a larger cohort.
Additionally, more children in HAPN lived on higher floors compared to children in LAPN and thus, may have been less affected by street-level pollutants compared to children in LAPN that lived on lower floors. Previously we have demonstrated that both the height of an urban building and the floor height that the child lives on can directly influence indoor concentrations of street-level pollutants; low-rise buildings and apartments on floors 0–2 have higher concentrations than homes on higher floors.(30) Although we had limited power for subgroup analysis, we observed a slight trend towards a greater association between NO2 and urgent asthma among children in HAPN that lived on lower floor apartment units. Thus, in NYC, annual outdoor pollutant exposure measured at the street-level may be a more important predictor of urgent medical visits for asthma among children living in LAPN homes because more children in LAPN live at or near the street-level. This association should be confirmed in a larger study population.
We observed higher concentrations of O3 in LAPN compared to HAPN and an unanticipated inverse relationship between O3 and urgent asthma visits in LAPN. Higher concentrations of other combustion related pollutants at street-side are known to result in lower concentrations of O3 within urban areas.(28) This may explain why O3 concentrations were lower in the more dense traffic, HAPNs. The negative association between O3 and urgent asthma visits in LAPN is likely confounded by traffic exposure. Hence, when we further controlled for summer average NO2 as an indicator of traffic pollution exposure, we no longer observed an association between O3 and urgent asthma visits in LAPN.
Although this study offers new insights into the relationship between exposure to pollutants and urgent asthma in an urban environment, it has several limitations. Street-level modeled concentrations of pollutants are not equivalent to personal or residential concentrations; therefore we have not fully accounted for individual exposure to all pollutants. However the geographically dense monitoring network and land use regression models that are part of the NYCCAS dataset provide fairly high spatial resolution for the modeled air pollutants and allow one to estimate annual trends. Like other cross sectional studies, ours was unable to demonstrate temporality and causality. In particular, our study design limited the ability to assess the temporal relationship between short-term spikes in pollution and urgent asthma visits. Similarly, our asthma outcome was assessed over the preceding 12 months included both summer and winter seasons, therefore it was impossible to disentangle the true impact of summer O3 concentration on summer urgent asthma visits. Also, the study may have been affected by non-differential misclassification as parents in HAPN may have been more likely than those in LAPN to recall asthma exacerbations. However, all the children had asthma, presumably a stronger risk factor for bias than neighborhood asthma prevalence. And to the extent that neighborhood prevalence resulted in bias, our results would have been biased towards the null hypothesis. While or case definition of asthma was intentionally broad thus capturing a potentially milder population of asthmatics, in sensitivity analysis among children with more frequent symptoms we observed similar relationships between pollutants and urgent asthma that differed by neighborhood asthma prevalence.
Overall, our findings support the link between high exposure to ambient pollutants and asthma exacerbations that require urgent medical care, especially in LAPN. The differences observed across adjacent neighborhoods may suggest a plateauing effect whereby increasing pollutant concentrations beyond a threshold contribute less to the risk of urgent asthma visits in HAPN. Thus, spatial variation in exposure to air pollution across adjacent neighborhoods within a city, coupled with other social and environmental factors, may lead to differing patterns of susceptibility in pediatric asthma. Given our findings in LAPN that had lower pollutant concentrations, efforts to improve air quality should be targeted at urban neighborhoods with both high and low exposure to pollutants to help address the disparity in pediatric asthma disease burden.
Supplementary Material
Acknowledgments
Funding Sources: KL2TR001874; NIEHS (R01 ES014400, P30 ES09089); HUD Healthy Homes Technical Studies (NYHHU0003-11); Harold Amos Medical Faculty Development Award Program – Robert Wood Johnson Foundation
Footnotes
Disclosure Statement: The authors have no relevant conflict of interests to disclose.
Study Category: clinical research
References:
- 1.Papi A, Brightling C, Pedersen SE, Reddel HK 2017. Asthma. Lancet [DOI] [PubMed]
- 2.Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW 1999. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 107:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strickland MJ, Darrow LA, Klein M, et al. 2010. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med 182:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villeneuve PJ, Chen L, Rowe BH, Coates F 2007. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health 6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore K, Neugebauer R, Lurmann F, et al. 2008. Ambient ozone concentrations cause increased hospitalizations for asthma in children: An 18-year study in Southern California. Environmental Health Perspectives 116:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gent JF, Triche EW, Holford TR, et al. 2003. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. Jama-Journal of the American Medical Association 290:1859–1867. [DOI] [PubMed] [Google Scholar]
- 7.Delfino RJ, Chang J, Wu J, et al. 2009. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol 102:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS, Kahn RS 2014. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr 164:1396–1402 e1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold DR, Wright R 2005. Population disparities in asthma. Annu Rev Public Health 26:89–113. [DOI] [PubMed] [Google Scholar]
- 10.Houston D, Wu J, Ong P, Winer A 2004. Structural disparities of urban traffic in Southern California: Implications for vehicle-related air pollution exposure in minority and high-poverty neighborhoods. Journal of Urban Affairs 26:565–592. [Google Scholar]
- 11.Milligan KL, Matsui E, Sharma H 2016. Asthma in Urban Children: Epidemiology, Environmental Risk Factors, and the Public Health Domain. Current Allergy and Asthma Reports 16. [DOI] [PubMed] [Google Scholar]
- 12.Patel MM, Quinn JW, Jung KH, et al. 2011. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res 111:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheirbek I, Wheeler K, Walters S, Kass D, Matte T 2013. PM2.5 and ozone health impacts and disparities in New York City: sensitivity to spatial and temporal resolution. Air Qual Atmos Health 6:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CH, Fan ZH, Lioy PJ, Baptista A, Greenberg M, Laumbach RJ 2016. A novel mobile monitoring approach to characterize spatial and temporal variation in traffic-related air pollutants in an urban community. Atmospheric Environment 141:161–173. [Google Scholar]
- 15.Patton AP, Perkins J, Zamore W, Levy JI, Brugge D, Durant JL 2014. Spatial and temporal differences in traffic-related air pollution in three urban neighborhoods near an interstate highway. Atmospheric Environment 99:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta RS, Zhang XY, Sharp LK, Shannon JJ, Weiss KB 2008. Geographic variability in childhood asthma prevalence in Chicago. Journal of Allergy and Clinical Immunology 121:639–645. [DOI] [PubMed] [Google Scholar]
- 17.Garg RKA, Leighton J, Perrin M, Shah M. Asthma Facts, Second Edition. New York City Department of Health and Mental Hygiene:https://www1.nyc.gov/assets/doh/downloads/pdf/asthma/facts.pdf. [Google Scholar]
- 18.Olmedo O, Goldstein IF, Acosta L, et al. 2011. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol 128:284–292 e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Leon Hsu HH, Mathilda Chiu YH, et al. 2017. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J Allergy Clin Immunol [DOI] [PMC free article] [PubMed]
- 20.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K 2009. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences of the United States of America 106:12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen E, Schreier HMC, Strunk RC, Brauer M 2008. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental Health Perspectives 116:970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clougherty JE, Levy JI, Kubzansky LD, et al. 2007. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental Health Perspectives 115:1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanc PD, Yen IH, Chen H, et al. 2006. Area-level socio-economic status and health status among adults with asthma and rhinitis. European Respiratory Journal 27:85–94. [DOI] [PubMed] [Google Scholar]
- 24.O’Lenick CR, Winquist A, Mulholland JA, et al. 2017. Assessment of neighbourhood-level socioeconomic status as a modifier of air pollution-asthma associations among children in Atlanta. Journal of Epidemiology and Community Health 71:129–136. [DOI] [PubMed] [Google Scholar]
- 25.Williams DR, Sternthal M, Wright RJ 2009. Social Determinants: Taking the Social Context of Asthma Seriously. Pediatrics 123:S174–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornell AG, Chillrud SN, Mellins RB, et al. 2012. Domestic airborne black carbon and exhaled nitric oxide in children in NYC. J Expo Sci Environ Epidemiol 22:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg R KA, Leighton J, Perrin M, Shah M May 2003. Asthma Facts In Hygiene NYCDoHaM; (ed). [Google Scholar]
- 28.Shmool JL, Kinnee E, Sheffield PE, Clougherty JE 2016. Spatio-temporal ozone variation in a case-crossover analysis of childhood asthma hospital visits in New York City. Environ Res 147:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maantay J 2007. Asthma and air pollution in the Bronx: methodological and data considerations in using GIS for environmental justice and health research. Health Place 13:32–56. [DOI] [PubMed] [Google Scholar]
- 30.Jung KH, Bernabe K, Moors K, et al. 2011. Effects of Floor Level and Building Type on Residential Levels of Outdoor and Indoor Polycyclic Aromatic Hydrocarbons, Black Carbon, and Particulate Matter in New York City. Atmosphere (Basel) 2:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tunnicliffe WS, Burge PS, Ayres JG 1994. Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet 344:1733–1736. [DOI] [PubMed] [Google Scholar]
- 32.Mainardi TR, Mellins RB, Miller RL, et al. 2013. Exercise-induced wheeze, urgent medical visits, and neighborhood asthma prevalence. Pediatrics 131:e127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.2017. The New York City Community Air Survey NYC Department of Health and Mental Hygiene. [Google Scholar]
- 34.Krieger J, Higgins DL 2002. Housing and health: time again for public health action. Am J Public Health 92:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


