Abstract
Inflammation and cellular energetics play critical roles in organ dysfunction following hemorrhagic shock. Recent studies suggest a putative role for sirtuin 1 (SIRT1) in potentiating mitochondrial function and improving organ function following hemorrhagic shock in animal models. SIRT1 is an NAD+ dependent protein deacetylase and increased availability of NAD+ has been shown to augment SIRT1 activity. As niacin is a precursor of NAD+, in this study, we tested whether niacin can improve survival following hemorrhagic shock. However niacin also mediates its biological action by binding to its receptor, hydroxylcarboxylic acid receptor 2 (HCA2 or Gpr109a); so we further asked whether the effect of niacin is mediated by binding to Gpr109a or by increasing NAD+ availability. We found that niacin administered intravenously to rats subjected to hemorrhagic injury (HI) in the absence of fluid resuscitation resulted in significantly prolonged duration of survival. However, treatment of rats with similar doses of nicotinamide mononucleotide (NMN), a precursor to NAD+ that does not bind Gpr109a, did not extend survival following HI. The duration of survival due to niacin treatment was significantly reduced in Gpr109a−/− mice subjected to HI. These experiments demonstrated that Gpr109a receptor-mediated pathway contributed significantly to niacin mediated salutary effect. Further studies showed improvement in markers of cellular energetics and attenuation of inflammatory response with niacin treatment. In conclusion, we report that Gpr109a-dependent signalling is important in restoring cellular energetics and immunometabolism following hemorrhagic shock.
Keywords: immunometabolism, nicotinic acid, trauma, hemorrhage
1. INTRODUCTION
Trauma is the leading cause of death in the United States and hemorrhagic shock accounts for 30%−40% of all trauma mortality [1]. Despite advances in critical care, severely injured patients still face a high degree of post-injury morbidity and mortality [2]. Hemorrhagic shock is characterized by whole body hypoxia, nutrient deprivation, hemodynamic instability and systemic inflammation, and may lead to multiple organ dysfunction and death [3, 4]. Studies from our laboratory and others have demonstrated a significant role for mitochondria in organ dysfunction following hemorrhagic shock [5–7]. SIRT1, an NAD+ dependent enzyme that catalyses protein deacetylation, is important in the maintenance of mitochondrial function and recent studies showed a declined activity of SIRT1 in animal models of hemorrhagic shock [4, 8]. We previously reported that resveratrol and SRT1720, SIRT1 activators, improved survival following hemorrhagic shock [9]. SIRT1 may also be activated indirectly by increasing intracellular level of nicotinamide adenine dinucleotide (NAD). As NAD is a metabolic by-product of niacin, and niacin supplementation is known to augment NAD levels [10–12], we hypothesized that treatment with niacin will improve organ function and survival following hemorrhagic shock.
Niacin (nicotinic acid) is a water-soluble vitamin participating in metabolic pathways as a precursor of NAD and NADP [13–15] and has been in clinical use in the treatment of plasma lipid disorders and atherosclerosis [16, 17]. Niacin has been shown to reduce systemic inflammation and oxidative stress [18, 19]. Niacin is also implicated in inflammasome activation in the gut promoting epithelial integrity[20].
Niacin is also known to exert physiological effects through the G-protein coupled receptor, hydroxyl-carboxylic acid receptor 2 (HCA2 or Gpr109a), also called niacin receptor [21–23]. The niacin binding to Gpr109a triggers a Gi-mediated adenylyl cyclase inhibition resulting in a decrease in intracellular cyclic AMP. Activation of Gpr109a leads to anti-lipophilic and anti-inflammatory effects through yet unidentified mechanisms. In this study we performed a systematic investigation of niacin mediated salutary effect and the role of Gpr109a in hemorrhagic shock.
2. MATERIALS AND METHODS
2.1. Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Augusta University and were performed in adherence to the NIH Guidelines on the Use of Laboratory Animals. Male Sprague Dawley rats of ages 10–12 weeks were obtained from Charles River Laboratory (Wilmington, MA, USA) and male C57BL/6 WT mice of 10–12 week old were obtained from Jackson laboratory. Gpr109a−/− mice (C57BL/6 background) [24] breeding colony were housed in Augusta University animal facility during the experiments as per the approved protocol.
2.2. Hemorrhagic shock.
The HI procedure was as described before [25]. The animals were anesthetized with 2.5% isoflurane (Henry Schein, Dublin, OH, USA) and soft tissue injury was induced by midline laparotomy in both sham and HI animals. In the rats femoral arteries and one femoral vein were cannulated, to monitor mean arterial pressure (MAP), bleeding and administration of fluids or drug. In the mice both femoral arteries were cannulated and fluid was administered through one of the arteries. Surgical sites were bathed with bupivacaine. Sham animals were not subjected to bleeding or resuscitation. The animals in HI groups were bled 60% to blood volume to a MAP of 40 ± 5 mmHg in rats and MAP of 35 ± 5 mmHg in mice. Resuscitation was performed with 2X Ringer’s lactate. Vehicle (Ringers lactate [RL]; 120 μL/rat and 50 μL mice) or drug was administered at 10 min from the end of shock period. The animals were euthanized three hours later and tissues removed. In survival studies, the experimental animals were randomly divided into different groups. Niacin, NMN or DMSO administered intravenously at the end of the shock period. To study molecular parameters a subset of animals were resuscitated by fluid. Animals that did not receive fluid resuscitation were observed for 6 hrs.
2.3. Determination of haematocrit and circulating lactate levels
Haematocrit was measured using a portable blood gas analysis instrument (Opti-Med, Atlanta, GA, USA). The plasma was separated and lactate levels were measured using a Lactate Assay Kit (Sigma st. Louis, MO 63103 USA) and expressed in relation to total plasma protein.
2.4. SIRT1 Activity Assay
The enzymatic activity of SIRT1 in the heart tissue was assayed by a fluorimetric assay by using the SensoLyte Green SIRT1 assay kit (AnaSpec, Fremont, CA, USA). The assay was performed according to the manufacturer’s directions. The acetylated p53 peptide substrate provided with the kit was incubated with Sirtuin containing tissue protein samples. Deacetylation of substrate sensitizes it to the color developer releasing the green fluorophore.
2.5. ATP Measurement
Total ATP in heart tissue was assayed by a bioluminescence assay (ATP determination kit; Invitrogen). Briefly, reaction solution containing luciferase and luciferin was plated and background luminescence measured. ATP standard solution or sample containing ATP was added to respective wells and luminescence was measured. ATP concentration was normalized to total protein concentration.
2.6. NAD assay
The ratio of NAD to NADH in heart tissue samples was determined using a colorimetric assay kit (Sigma MAK037). To differentiate between NADH and total NAD, a control for each sample was heated to 60° C for 30 minutes to decompose NAD. After a 2 hour incubation with NAD cycling enzyme at room temperature, absorbance was read at 450nm and compared against a NADH standard. The NAD/NADH ratio was then calculated for each sample, and normalized to the sham values for each experiment.
2.7. Western Blot Analysis
The heart tissues were homogenized in Pierce RIPA lysis buffer (Thermo Scientific, Chicago, IL) and tissue lysates were centrifuged, the supernatant saved for protein estimation and analysis. Protein aliquots were mixed with Laemmli buffer (Bio-Rad, Hercules, CA, USA) and resolved on a 10% SDS polyacrylamide gel, transferred to PVDF membrane, blocked for 1 h at room temperature (RT). The membranes were probed with primary antibodies. The primary antibodies to c-Myc (cat No. #sc40 Mouse mAb; (Santa cruz), P-NFKb (cat No. #, (Ser536) (93H1) Rabbit mAb 3033; (Cell Signaling Technology) NFKb (cat No. # NF-κB p65 (D14E12) Rabbit mAb 8242; (Cell Signaling Technology), HMGB1 (cat No. # (D3E5) Rabbit mAb #6893; (Cell Signaling Technology)) and GAPDH (cat No. # (14C10) Rabbit mAb #2118 ) were used. After overnight incubation the membranes were treated with horseradish peroxidase conjugated secondary antibody for 1 h at RT and developed using enhanced western lightning plus-ECL (cat No. #NEL105001EA; (PerkinElmer). Protein bands were quantified using the ImageJ software (Wayne Rasband, NIH, Rockville, MD, USA).
2.8. Real-time Polymerase Chain Reaction
Total RNA was isolated from heart tissue using total RNA isolation TRIzol™ Reagent (Cat no. #15596026; (Invitrogen™) according to the manufacturer’s protocol and cDNA synthesized (Agilent Technologies, CA). Total RNA (200 ng) was reverse transcribed to cDNA using reverse transcription kit (Promega)[26]. Quantitative real-time PCR was performed using Agilent Technologies Stratagene Mx3000P real-time PCR machine. The primer sequences were: β-actin, TTCTACAATGAG (F), GGGGTGTTGAAGGTCTCAAA (R); IFNβ, CACGCCGCGTCTTGGT (F) TCTAGGCTTTCAATGAGTGTGCC (R); IL-6, GAGCCCACCAGGAACGAAA (F), AACTGGCTGGAAGTCTCTTGC (R); IL-10, GTTGCCAAGCCTTGTCAGAAA (F), TTTCTGGGCCATGGTTCTCT (R); IL1β, CCCTGCAGCTGGAGAGTGTGG (F), TGTGCTCTGCTTGAGAGGTGCT (R); and, IL18, CAGACCACTTTGGCAGACTTCACT (F), GGATTCGTTGGCTGTTCGGTCG (R).The thermal cycling conditions were 95°C for 5 min followed by 40 cycles of 95°C for 15 seconds and 60°C for 50 seconds. Results are expressed as a ratio of expression to beta actin and normalized to the values obtained for samples in sham group.
2.9. Measurement of intestinal permeability
Intestinal permeability was evaluated in terms of the amount of FITC-dextran that crossed the intestinal epithelial barrier into the blood after oral ingestion. At the end of fluid resuscitation the mice were gavaged with 0.4ml of 22mg/ml FITC-dextran in PBS. After 2 hours blood was drawn, and the mice were sacrificed and small intestine collected. The plasma was separated from the blood, and read spectrophotometrically at 480nm excitation and 520nm emission wavelengths for FITC fluorescence. To observe FITC absorption in intestinal tissue, cryosections of intestine were made in the dark and examined under a confocal microscope.
2.10. Statistics
Survival analysis was performed by Kaplan-Meier plot and significance between survival curves was determined using GraphPad software (La Jolla, CA). Multigroup comparisons were carried out and significance determined by one-way ANOVA followed by Tukey test using GraphPad software. Two-group comparisons for significance were done by unpaired t test using GraphPad software.
3. RESULTS
3.1. Niacin improves survival in a rat model of hemorrhagic shock
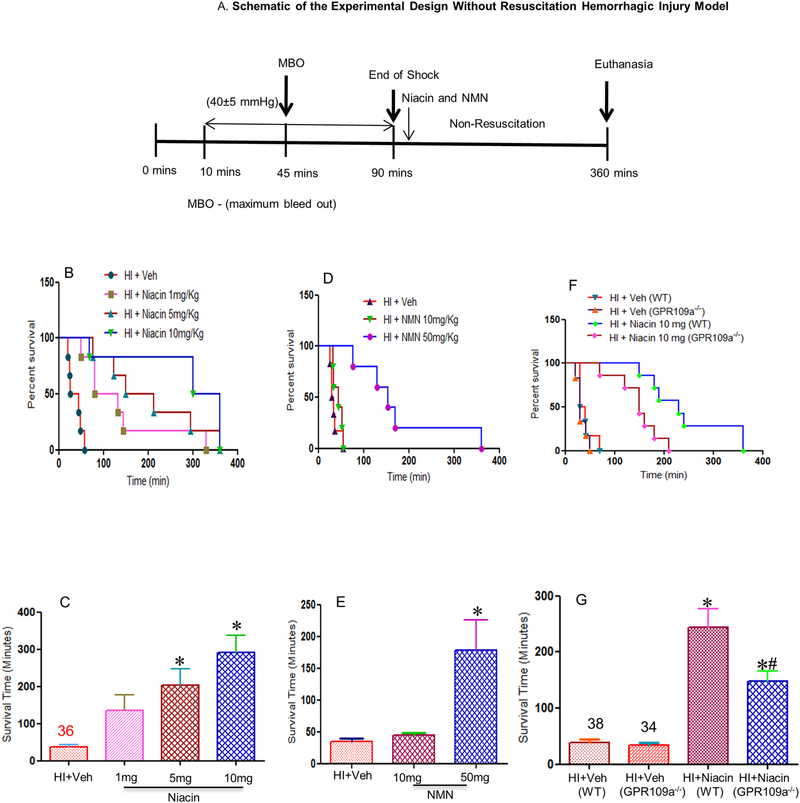
Our first experiment was to test the effectiveness and dose dependence of niacin in HI. We tested three different doses of niacin: 1 mg, 5 mg and 10 mg per Kg body weight, in rats. Among the doses tested, 10 mg/Kg dose demonstrated the best survival response (Figure 1A, B). The mean survival times were 36 min, 136 min, 203 min and 291 min for doses 0, 1, 5 and 10 mg/Kg/body weight respectively (Figure 1B, C). All the tested doses showed a significant improvement in survival. These results show that niacin when administered intravenously following hemorrhagic shock can significantly prolong life.
Figure 1: Effect of niacin on survival following HI.
(A) Schematic diagram of the experimental design of HI model without fluid resuscitation. (B-E) Niacin prolongs life in rats after HI in the absence of fluid resuscitation. Kaplan-Meier curves (B,D) show survival rate. Groups in panel (B): HI + Veh (n=6), HI + Niacin 1mg/Kg (n=6), HI + Niacin 5 mg/Kg (n=6), HI + Niacin 10mg/Kg (n=6); panel (C): HI + Veh (n=6), NMN 50mg/Kg (n=5) and NMN 10mg/Kg (n=5). (F,G) GPR109a−/− is necessary for niacin-mediated improved survival after HI in the absence of fluid resuscitation. Kaplan-Meier (F) survival curves show survival rate. HI + Veh (WT and GPR109a−/−mice), HI + Niacin 10 mg/Kg (WT and GPR109a−/−mice); n=7. Bars=Mean+SE *indicates p<0.05 compared to HI + Veh. #indicates p<0.05 for HI + Niacin WT compared to HI + Niacin GPR109a−/−.
3.2. Role of NAD+ vs HCA2 in niacin mediated salutary effect
Though the salutary effect of niacin is known to be due to its metabolite, NAD+, the effect may also be mediated through its non-cognate receptor Gpr109a (HCA2), called niacin receptor. Humans have two isoforms of niacin receptor: Gpr109a and Gpr109b, whereas rodents have only one, Gpr109a [27, 28]. To determine whether the beneficial effect of niacin was due to its interaction with Gpr109a, we treated rats with nicotinamide mononucleotide (NMN) which does not bind Gpr109a but metabolizes to NAD+ (Figure 1D,E). We observed a significantly decreased duration of survival following NMN treatment as compared to niacin treatment. In order to obtain a survivability similar to that was achieved by niacin, five times higher dose of NMN was needed, indicating that Gpr109a plays an important role in niacin mediated salutary effect.
3.3. Contribution of HCA2 in niacin mediated effect using HCA2 deficient mice
To further establish the role of Gpr109a in niacin mediated survival we used a mouse model of hemorrhagic shock. We used Gpr109a−/− mice that has been previously characterized [24, 29]. As shown in Figure 1F and G, average survival rate for untreated WT and Gpr109a−/− mice were 38 and 34 minutes respectively. The survival rate in WT mice was similar to that observed in the rat model. However, WT mice treated with niacin survived for 244 minutes (mean), whereas Gpr109a−/− mice survived only 148 minutes (mean), significantly shorter duration than the WT mice. This demonstrates that in the absence of Gpr109a, effectiveness of niacin was significantly reduced, establishing a critical role for Gpr109a in niacin mediated effect in the outcome following HI.
3.4. Niacin improved physiological function following HI.
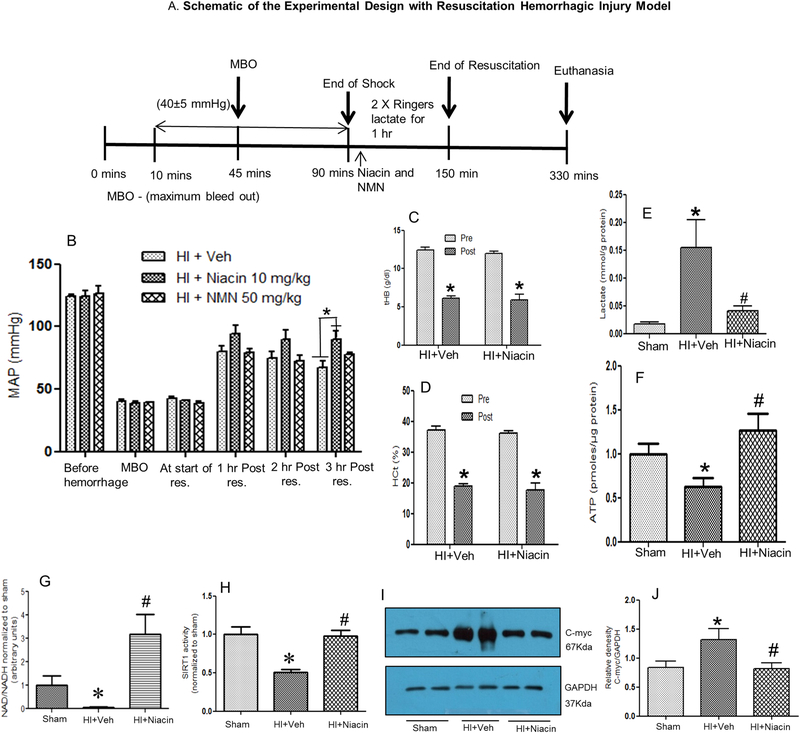
In order to obtain a mechanistic understanding of the effect of niacin on HI at the physiological and molecular level, a subset of animals underwent fluid resuscitation following shock (See Figure 2A,B). In this model the animals were sacrificed at 3 hrs following the end of resuscitation. As shown in Figure 2B, fluid resuscitation significantly improved MAP at 1, 2 and 3 hours following hemorrhage and shock. The group of animals that received niacin demonstrated a consistently increased MAP compared to Veh treated animals, and the increase was significant at 3 hours after resuscitation. The MAP in the mice that received NMN showed a pattern of MAP similar to the Veh treated animals. The blood loss following hemorrhage is illustrated in Figures 3C and D illustrating marked decrease in hematocrit and total haemoglobin post-HI.
Figure 2: Cellular energetics following HI and niacin treatment.
(A) Schematic diagram of the experimental design of HI model with fluid resuscitation. (B) Mean arterial pressure (MAP) following HI. MAP before hemorrhage, at maximum bleed out (MBO), at the start of resuscitation and at 1, 2 and 3 hours post-resuscitation. Groups: HI + Veh (n=7), HI + Niacin 10 mg/Kg (n=7) and HI + NMN 50 mg/Kg (n=5). Bars=Mean+SE. *p<0.05. (C,D) Total haemoglobin (tHB) and hematocrit (HCt) values before (pre) and after (post) hemorrhage in treated and untreated groups demonstrating bleedout. Bars=Mean+SEM, * indicates p<0.05 compared to respective pre-hemorrhage values. (E-H) Plasma lactate; ATP, NAD, Sirt-1 activity and (I,J) c-Myc expression in the heart for sham (n=6), HI+Veh (n=6) and HI + Niacin (n=6). * indicates p<0.05 compared to Sham and #indicates p<0.05 compared to HI+Veh.
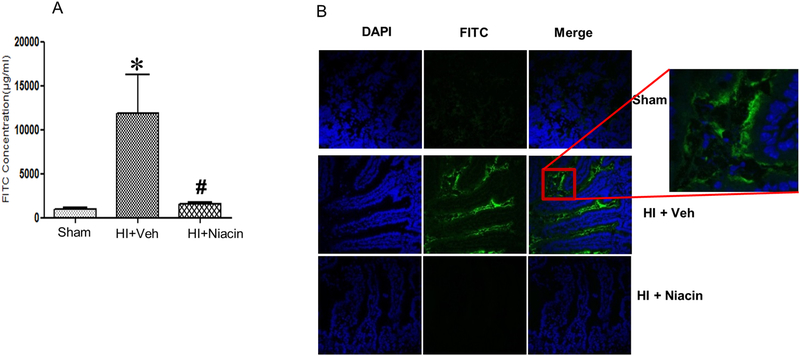
Figure 3. Intestinal permeability following HI and niacin treatment.
(A) Intestinal barrier permeability measured by determining plasma concentration of FITC fluorescence levels in sham, HI+Veh and HI+ Niacin groups, after oral gavage with FITC-dextran; (n=3–4); *indicates p<0.05 compared to HI + Veh and #indicates p<0.05 compared to HI + Niacin. (B) FITC fluorescence in cryosections of small intestine by confocal microscopy. DAPI, FITC and merged figures.
3.5. Niacin improved cellular energetics
The hypoxic condition following hemorrhagic shock and recovery with niacin treatment is evident in the lactate levels in these animals (Figure 2E). The near-sham level of lactate in animals treated with niacin indicates a productive glycolysis, as opposed to lactate level in untreated animals. The impairment of cellular energetics is a hall mark of hemorrhagic shock. The elevated lactate levels following HI shown in Figure 2E is consistent with the mitochondrial-glycolytic shift observed in metabolism following HI. This is further confirmed by a decreased tissue ATP (Figure 2F) in the heart after HI and increase with niacin treatment suggesting that niacin treatment facilitated a normalized cellular energetics. Cellular metabolic status is indicated by NAD+/NADH ratio[30] and the results demonstrate restoration of the levels by niacin (Figure 2G). Furthermore, the activity of SIRT1, a deacetylase critical in the function of mitochondria, declined in the heart after HI and was restored with niacin treatment (Figure 2H). The increased c-Myc following HI and its restoration with niacin treatment further demonstrates niacin-mediated mitochondrial homeostasis (Figure 2I, J).
3.6. Intestinal permeability
The intestinal permeability assay was carried by oral gavage of dextran-FITC conjugate and assessing the FITC fluorescence in the plasma. The fluoro tag was administered immediately after resuscitation and plasma tested two hours later. As seen in Figure 3A, there was a significant increase in barrier permeability in vehicle treated group which is demonstrated by the increased plasma FITC concentration, whereas permeability was restored in niacin treated group. This result was further confirmed by examining the cryosections of the small intestine for FITC fluorescence by confocal microscopy. When green fluorescence was widely present in the villi of intestinal tissues harvested from vehicle treated animals, tissues from niacin treated animals did not exhibit absorption of green fluorescence (Fig 3B).
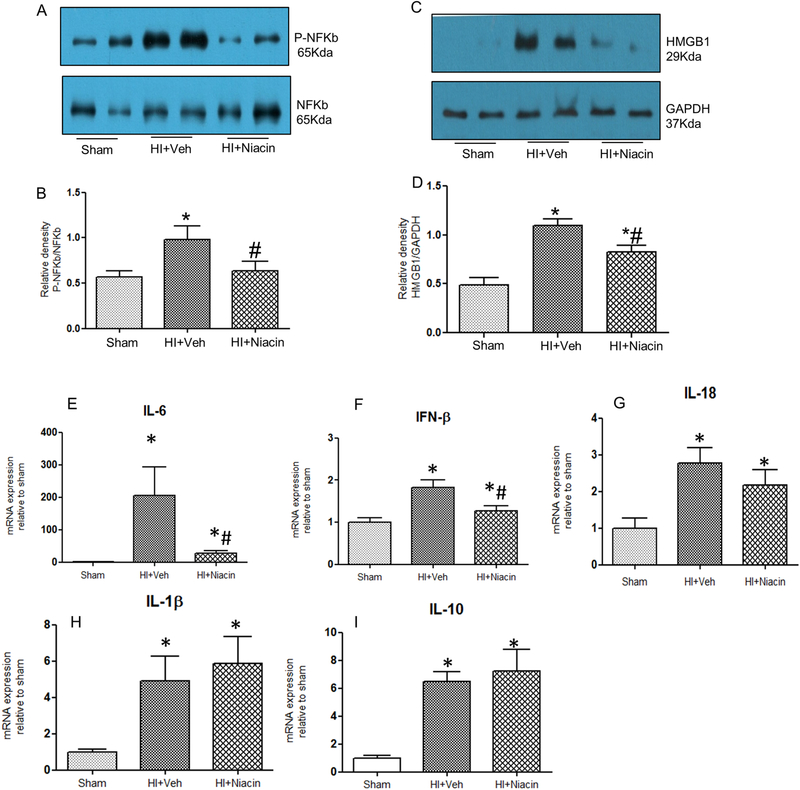
3.7. Effect of niacin on inflammatory response
There was a significant decrease in the level of phosphorylated p65, NFk-b subunit, and the level was normalized following niacin treatment. The expression changes in HMGB1, IL6, IF1-β, IL18, IFN-β and IL-10 also demonstrated the strong inflammatory response following HI and attenuation of the expression of Il-6, IFN-b and IL-18 with niacin treatment (Figure 4A-I).
Figure 4. Inflammatory response following HI and niacin treatment in heart tissue samples.
(A-D) p-NF-kb/NF-kb ratio and HMGB1 protein expression following HI and niacin treatment. (n=7); *indicates p<0.05 compared to Sham. # indicates p<0.05 compared to HI + Veh. (E-I) IL6, IFN-β, IL18, IL1-β and IL10 mRNA expression following HI and niacin treatment assessed by real time PCR. n=6 in each group; *indicates p<0.05 compared to Sham; # indicates p<0.05 compared to HI +Veh.
4. DISCUSSION
Hemorrhagic shock following significant blood loss leads to decreased oxygen delivery, tissue hypoxia, hemodynamic instability, multiple organ dysfunction and death [31, 32]. Cellular energetics play a major role in outcome following hemorrhagic shock. Several studies demonstrated that mitochondrial functional decline contributes to imbalance in cellular homeostasis following hemorrhagic shock and the protein deacetylase SIRT1 has been shown to play an important role in maintaining mitochondrial function [9, 25]. The SIRT1 mediated deacetylation regulates a number of transcription factors including Pgc-1α, NF-κb, FOXO1 and p53 [33]. SIRT1 is an NAD+ dependent enzyme that catalyzes deacetylation of target proteins and augmentation of the cofactor NAD+ has been shown to increase the activity of SIRT1[34]. NAD+ is one of the metabolic products of niacin, and exogenous administration of niacin has been shown to increase intracellular NAD+.
Our data show that administration of niacin, a precursor of NAD+, improves survival following HI. However, the salutary effect of niacin may also be mediated through its non-cognate receptor Gpr109a, also called niacin receptor. Humans have two isoforms of niacin receptor: GPR109a and GPR109b, whereas rodents have only one, Gpr109a [27, 28]. In order to determine whether the beneficial effect of niacin was due to its binding to Gpr109a, we treated rats with nicotinamide mononucleotide (NMN) which does not bind Gpr109a but metabolizes to NAD+. In the rats, even at 5-fold higher dose, NMN was unable to increase survival rate to the level observed in niacin treated mice. This result showed a potential role of Gpr109a in niacin-mediated survival following HI. In order to further establish the role of Gpr109a, we tested the effect of niacin in mice deficient in Gpr109a and observed a significant decrease in mean survival duration. These experiments clearly demonstrate that Gpr109a contributes to niacin mediated salutary effect in hemorrhagic shock.
In order to obtain a mechanistic understanding of the effect of niacin on hemorrhagic shock, a subset of animals underwent fluid resuscitation following shock and were euthanized three hours later. In these animals, niacin improved NAD+/NADH ratio, SIRT1 activity, and total tissue ATP level. Though the exquisite specificity of the assay to SIRT1 is not known, increased sirtuin activity nevertheless demonstrates metabolic significance. Furthermore, cellular metabolic status is indicated by NAD+/NADH ratio[30] and our results demonstrate metabolic potentiation following niacin administration. Consistent with this, in previously published studies we showed that directly activating mitochondria by targeting pyruvate dehydrogenase kinase (Pdk) with dichloroacetate (DCA), outcome following hemorrhagic shock can be improved[25]. Additional studies show improved systemic lactate levels, reduced proinflammatory cytokines in the heart and restoration of intestinal barrier permeability following niacin treatment.
The compromised intestinal barrier permeability due to decreased organ perfusion following hemorrhagic shock has been described before [35, 36]. The barrier disruption leads to dysregulation of barrier function as well as translocation of luminal peptides and bacteria [35, 36]. It has also been suggested that splanchnic gut injury may lead to the generation of biologically active factors in intestinal lymph [36]. These effects contribute to shock and organ dysfunction.
Niacin has been known to exert anti-inflammatory, anti-lipolytic and antioxidative properties [18, 19, 37]. It has been shown to have clinical benefits and improve blood pressure and endothelial dysfunction in several animal models [37–40]. There was a recent report indicating that niacin may reduce lung injury in a rat model of hemorrhagic shock at a very high oral dose of 1180 mg/Kg [37]. However, its effectiveness through intravenous use or the contribution of Gpr109a in niacin-mediated effects is unknown. Nevertheless, it has been demonstrated that signal transduction through Gpr109a is anti-inflammatory. The anti-inflammatory effect of Gpr109a mediated signalling was recently reported in a colitis model wherein suppression of inflammatory response of antigen presenting cells induced Treg differentiation [29, 41]. The authors further showed that Gpr109a is essential for the expression of IL-18 in colonic epithelium and Gpr109a deficiency increased susceptibility to colitis in mice [29]. It is also shown that colonic levels of TNF-α, VEGF, angiostatin and endostatin can be reversed by niacin[42].
Our experiments show that the salutary effect due to niacin is not only due to its metabolism to NAD+, but principally as a ligand of Gpr109a. Though the mechanism of action of niacin through its metabolite NAD+ involves regulation of cellular energetics through activation of SIRT1 and mitochondrial function, and Gpr109a-mediated effect is primarily Gi mediated, it is unclear whether there is a convergence of these two pathways [43]. Our results demonstrate that augmentation of NAD+ together with activation of HCA2 enhance the salutary effect. These results may also have implications in methods that enhance NAD+ by supplementing NMN or nicotinamide riboside to potentiate mitochondrial function.
Hemorrhagic shock is a complex multi-hit process affecting a number of regulatory factors in cellular metabolism and energy homeostasis. This is the first demonstration that Gpr109a regulation plays a role in modulating outcome following hemorrhagic shock. Though precise downstream mediators of Gpr109a are unclear, the results demonstrate that niacin can used an adjunct to resuscitation fluid.
Highlights.
Niacin improved organ function and survival following hemorrhagic shock.
Niacin was more effective than nicotinamide mononucleotide (NMN) in improving survival.
Salutary effect of niacin in hemorrhagic shock is HCA2 dependent.
Acknowledgements:
The study was supported by NIH grants R01 GM122059 and R01 GM101927 to RR and R01DK103576 to NS. The authors are thankful to Dr. Stefen Offermanns at Max Planck Institute for Heart and Lung Research for GPR109a knock out mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kauvar DS, Lefering R, Wade CE, Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations, The Journal of trauma, 60 (2006) S3–11. [DOI] [PubMed] [Google Scholar]
- [2].Pfeifer, Patterns of mortality and causes of death in polytrauma patients—Has anything changed?, Injury., 40 (2009) 907. [DOI] [PubMed] [Google Scholar]
- [3].Tsukamoto Takeshi, Current theories on the pathophysiology of multiple organ failure after trauma, Injury., 41 (2010) 21–26. [DOI] [PubMed] [Google Scholar]
- [4].Lee MY, Yang DK, Kim SJ, Alterations of Mg(2+) After Hemorrhagic Shock, Biological trace element research, 180 (2017) 120–126. [DOI] [PubMed] [Google Scholar]
- [5].Jian B, Yang S, Chaudry IH, Raju R, Resveratrol improves cardiac contractility following trauma-hemorrhage by modulating Sirt1, Molecular medicine, 18 (2012) 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Villarroel JP, Guan Y, Werlin E, Selak MA, Becker LB, Sims CA, Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression, The journal of trauma and acute care surgery, 75 (2013) 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Warren M, Subramani K, Schwartz R, Raju R, Mitochondrial dysfunction in rat splenocytes following hemorrhagic shock, Biochimica et biophysica acta, 1863 (2017) 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeng Z, Chen Z, Xu S, Song R, Yang H, Zhao KS, Polydatin Alleviates Small Intestine Injury during Hemorrhagic Shock as a SIRT1 Activator, Oxid Med Cell Longev, 2015 (2015) 965961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jian B, Yang S, Chaudry IH, Raju R, Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury in a rat model, Molecular medicine, 20 (2014) 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stein LR, Imai S, The dynamic regulation of NAD metabolism in mitochondria, Trends Endocrinol Metab, 23 (2012) 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Si Y, Zhang Y, Zhao J, Guo S, Zhai L, Yao S, Sang H, Yang N, Song G, Gu J, Qin S, Niacin inhibits vascular inflammation via downregulating nuclear transcription factor-kappaB signaling pathway, Mediators of inflammation, 2014 (2014) 263786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sauve AA, NAD+ and vitamin B3: from metabolism to therapies, J Pharmacol Exp Ther, 324 (2008) 883–893. [DOI] [PubMed] [Google Scholar]
- [13].Bogan KL, Brenner C, Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition, Annual review of nutrition, 28 (2008) 115–130. [DOI] [PubMed] [Google Scholar]
- [14].Jonsson BH, Nicotinic Acid Long-Term Effectiveness in a Patient with Bipolar Type II Disorder: A Case of Vitamin Dependency, Nutrients, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Drueke TB, Massy ZA, Lowering Expectations with Niacin Treatment for CKDMBD, Clin J Am Soc Nephrol, 13 (2018) 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tavintharan S, Lim SC, Sum CF, Effects of niacin on cell adhesion and early atherogenesis: biochemical and functional findings in endothelial cells, Basic & clinical pharmacology & toxicology, 104 (2009) 206–210. [DOI] [PubMed] [Google Scholar]
- [17].Meyers CD, Kamanna VS, Kashyap ML, Niacin therapy in atherosclerosis, Current opinion in lipidology, 15 (2004) 659–665. [DOI] [PubMed] [Google Scholar]
- [18].Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelstiel CD, Karas RH, Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease, The American journal of cardiology, 98 (2006) 743–745. [DOI] [PubMed] [Google Scholar]
- [19].Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML, Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells, Atherosclerosis, 202 (2009) 68–75. [DOI] [PubMed] [Google Scholar]
- [20].Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, McKenzie C. Ian, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR, Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome, Nat Commun, 6 (2015) 6734. [DOI] [PubMed] [Google Scholar]
- [21].Chai JT, Digby JE, Choudhury RP, GPR109A and vascular inflammation, Curr Atheroscler Rep, 15 (2013) 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pike NB, Wise A, Identification of a nicotinic acid receptor: is this the molecular target for the oldest lipid-lowering drug?, Curr Opin Investig Drugs, 5 (2004) 271–275. [PubMed] [Google Scholar]
- [23].Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K, Molecular identification of nicotinic acid receptor, Biochem Biophys Res Commun, 303 (2003) 364–369. [DOI] [PubMed] [Google Scholar]
- [24].Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S, PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect, Nat Med, 9 (2003) 352–355. [DOI] [PubMed] [Google Scholar]
- [25].Subramani K, Lu S, Warren M, Chu X, Toque HA, Caldwell RW, Diamond MP, Raju R, Mitochondrial targeting by dichloroacetate improves outcome following hemorrhagic shock, Scientific reports, 7 (2017) 2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rio DC, Ares M Jr., Hannon GJ, Nilsen TW, Purification of RNA using TRIzol (TRI reagent), Cold Spring Harb Protoc, 2010 (2010) [DOI] [PubMed] [Google Scholar]
- [27].Ahmed K, Biological roles and therapeutic potential of hydroxy-carboxylic Acid receptors, Front Endocrinol (Lausanne), 2 (2011) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ahmed K, Tunaru S, Offermanns S, GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors, Trends Pharmacol Sci, 30 (2009) 557–562. [DOI] [PubMed] [Google Scholar]
- [29].Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V, Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis, Immunity, 40 (2014) 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Canto C, Menzies KJ, Auwerx J, NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus, Cell Metab, 22 (2015) 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Filho IT, Hemorrhagic Shock and the Microvasculature, Compr Physiol, 8 (2017) 61–101. [DOI] [PubMed] [Google Scholar]
- [32].Gutierrez G, Reines HD, Wulf-Gutierrez ME, Clinical review: hemorrhagic shock, Crit Care, 8 (2004) 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Olmos Y, Sanchez-Gomez FJ, Wild B, Garcia-Quintans N, Cabezudo S, Lamas S, Monsalve M, SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex, Antioxid Redox Signal, 19 (2013) 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Poulose N, Raju R, Sirtuin regulation in aging and injury, Biochimica et biophysica acta, 1852 (2015) 2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alsaigh T, Chang M, Richter M, Mazor R, Kistler EB, In vivo analysis of intestinal permeability following hemorrhagic shock, World J Crit Care Med, 4 (2015) 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Deitch EA, Senthil M, Brown M, Caputo F, Watkins A, Anjaria D, Badami C, Pisarenko V, Doucet D, Lu Q, Feketeova E, Xu DZ, Trauma-shock-induced gut injury and the production of biologically active intestinal lymph is abrogated by castration in a large animal porcine model, Shock, 30 (2008) 135–141. [DOI] [PubMed] [Google Scholar]
- [37].Jeong KY, Suh GJ, Kwon WY, Kim KS, Jung YS, Kye YC, The therapeutic effect and mechanism of niacin on acute lung injury in a rat model of hemorrhagic shock: Down-regulation of the reactive oxygen species-dependent nuclear factor kappaB pathway, The journal of trauma and acute care surgery, 79 (2015) 247–255. [DOI] [PubMed] [Google Scholar]
- [38].Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, Schinzel R, Walter U, Peetz D, Lackner K, Blankenberg S, Munzel T, Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study, Atherosclerosis, 204 (2009) 216–221. [DOI] [PubMed] [Google Scholar]
- [39].Klein AH, Wendroth SM, Drewes LR, Andrews MT, Small-volume d-beta-hydroxybutyrate solution infusion increases survivability of lethal hemorrhagic shock in rats, Shock, 34 (2010) 565–572. [DOI] [PubMed] [Google Scholar]
- [40].Lin T, Koustova E, Chen H, Rhee PM, Kirkpatrick J, Alam HB, Energy substrate-supplemented resuscitation affects brain monocarboxylate transporter levels and gliosis in a rat model of hemorrhagic shock, The Journal of trauma, 59 (2005) 1191–1202; [DOI] [PubMed] [Google Scholar]
- [41].Jobin C, GPR109a: the missing link between microbiome and good health?, Immunity, 40 (2014) 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Salem HA, Wadie W, Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis, Scientific reports, 7 (2017) 7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tang BL, Sirt1 and the Mitochondria, Mol Cells, 39 (2016) 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]