Fig. 1.
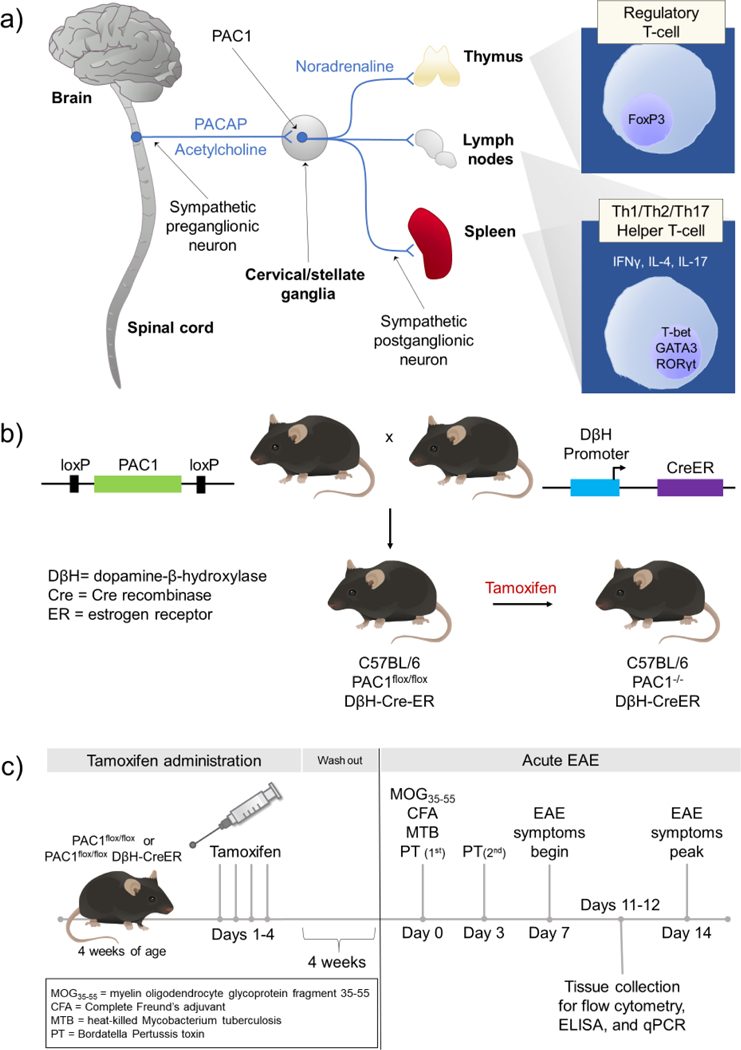
(a) Potential neural circuitry by which PACAP modulates peripheral immune cell activity during inflammatory stress. During inflammatory stress, neurons in the brain stem and hypothalamus activate preganglionic sympathetic neurons in the spinal cord. PACAP is expressed along with acetylcholine in the preganglionic neurons in thoracic spinal cord. When released during stress or inflammation, PACAP acts via PAC1 receptors expressed on sympathetic neurons in cervical/stellate ganglia to alter the immune response in the thymus, lymph nodes, and spleen. (b) Approach to conditionally knockout PAC1 receptors in postganglionic neurons in the SNS. C57BL/6 mice were genetically engineered to introduce loxP sites that flank critical sequences in the PAC1 receptor gene. Such mice (PAC1flox/flox mice) are bred to C57BL/6 mice which express Cre enzyme physically linked to the ligand-binding portion of the estrogen receptor driven by the dopamine β-hydroxylase (DβH) promoter (DβH-CreER). Administration of tamoxifen to PAC1flox/flox DβH-CreER mice allows Cre recombinase to translocate into the nucleus specifically in DβH-expressing cells, and subsequently disrupt the endogenous PAC1 receptor gene. (c) Experimental timeline to conditionally knockout PAC1 and induce EAE. PAC1flox/flox and PAC1flox/flox DβH Cre-ER mice are administered tamoxifen (Sigma Aldrich, St. Louis, MO, USA) (100 μL of 20 mg/mL dissolved in peanut oil) by daily oral gavage for four consecutive days at 4 weeks of age, the age at which the pups are weaned. Because tamoxifen is a known estrogen receptor agonist, we allow four weeks for tamoxifen washout to minimize any confounding effects of tamoxifen before proceeding with immunization to MOG35–55.
